Genetic immunization elicits antigen-specific protective immune responses and decreases disease severity in Trypanosoma cruzi infection
- PMID: 12228281
- PMCID: PMC128309
- DOI: 10.1128/IAI.70.10.5547-5555.2002
Genetic immunization elicits antigen-specific protective immune responses and decreases disease severity in Trypanosoma cruzi infection
Abstract
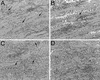
Immunity to Trypanosoma cruzi requires elicitation of humoral and cell-mediated immune responses to extracellular trypomastigotes and intracellular amastigotes. In this study, the effectiveness of the T. cruzi trans-sialidase family (ts) genes ASP-1, ASP-2, and TSA-1 as genetic vaccines was assessed. Immunization of mice with plasmids encoding ASP-1, ASP-2, or TSA-1 elicited poor antigen-specific cytotoxic-T-lymphocyte (CTL) activity and T. cruzi-specific antibody responses. Codelivery of interleukin-12 and granulocyte-macrophage colony-stimulating factor plasmids with antigen-encoding plasmids resulted in a substantial increase in CTL activity and antibody production and in increased resistance to T. cruzi infection. In pooled results from two to four experiments, 30 to 60% of mice immunized with antigen-encoding plasmids and 60 to 80% of mice immunized with antigen-encoding plasmids plus cytokine adjuvants survived a lethal challenge with T. cruzi. In comparison, 90% of control mice injected with empty plasmid DNA died during the acute phase of infection. However, the pool of three ts genes provided no greater protection than the most effective single gene (ASP-2) either with or without coadministration of cytokine plasmids. Importantly, the extent of tissue parasitism, inflammation, and associated tissue damage in skeletal muscles during the chronic phase of T. cruzi infection in mice immunized with antigen-encoding plasmids plus cytokine adjuvants was remarkably reduced compared to mice immunized with only cytokine adjuvants or empty plasmid DNA. These results identify new vaccine candidates and establish some of the methodologies that might be needed to develop effective vaccine-mediated control of T. cruzi infection. In addition, this work provides the first evidence that prophylactic genetic immunization can prevent the development of Chagas' disease.
Figures


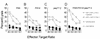



References
-
- Alberti, E., A. Acosta, M. E. Sarmiento, C. Hidalgo, T. Vidal, A. Fachado, L. Fonte, L. Izquierdo, J. F. Infante, C. M. Finlay, and G. Sierra. 1998. Specific cellular and humoral immune response in BALB/c mice immunized with an expression genomic library of Trypanosoma cruzi. Vaccine 16:608-612. - PubMed
-
- Bliss, J., V. Van Cleave, K. Murray, A. Wiencis, M. Ketchum, R. Maylor, T. Haire, C. Resmini, A. K. Abbas, and S. F. Wolf. 1996. IL-12, as an adjuvant, promotes a T helper 1 cell, but does not suppress a T helper 2 cell recall response. J. Immunol. 156:887-894. - PubMed
-
- Brener, Z., and R. T. Gazzinelli. 1997. Immunological control of Trypanosoma cruzi infection and pathogenesis of Chagas' disease. Int. Arch. Allergy Immunol. 114:103-110. - PubMed
-
- Costa, F., G. Franchin, V. L. Pereira-Chioccola, M. Ribeirao, S. Schenkman, and M. M. Rodrigues. 1998. Immunization with a plasmid DNA containing the gene of trans-sialidase reduces Trypanosoma cruzi infection in mice. Vaccine 16:768-774. - PubMed
-
- Cox, F. E. 1997. Designer vaccines for parasitic diseases. Int. J. Parasitol. 27:1147-1157. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

