Legionella pneumophila feoAB promotes ferrous iron uptake and intracellular infection
- PMID: 12228295
- PMCID: PMC128349
- DOI: 10.1128/IAI.70.10.5659-5669.2002
Legionella pneumophila feoAB promotes ferrous iron uptake and intracellular infection
Abstract
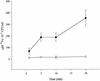
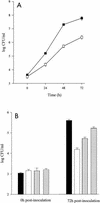
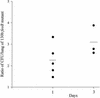
In order to determine the role of ferrous iron transport in Legionella pathogenesis, we identified and mutated the feoB gene in virulent Legionella pneumophila strain 130b. As it is in Escherichia coli, the L. pneumophila feoB gene was contained within a putative feoAB operon. L. pneumophila feoB insertion mutants exhibited decreased ferrous but not ferric iron uptake compared to the wild type. Growth on standard buffered charcoal yeast extract agar or buffered yeast extract broth was unaffected by the loss of L. pneumophila FeoB. However, the L. pneumophila feoB mutant had a reduced ability to grow on buffered charcoal yeast extract agar with a reduced amount of its usual iron supplementation, a phenotype that could be complemented by the addition of feoB in trans. In unsupplemented buffered yeast extract broth, the feoB mutant also had a growth defect, which was further exacerbated by the addition of the ferrous iron chelator, 2,2'-dipyridyl. The feoB mutant was also 2.5 logs more resistant to streptonigrin than wild-type 130b, confirming its decreased ability to acquire iron during extracellular growth. Decreased replication of the feoB mutant was noted within iron-depleted Hartmannella vermiformis amoebae and human U937 cell macrophages. The reduced intracellular infectivity of the feoB mutant was complemented by the introduction of a plasmid containing feoAB. The L. pneumophila feoB gene conferred a modest growth advantage for the wild type over the mutant in a competition assay within the lungs of A/J mice. Taken together, these results indicate that L. pneumophila FeoB is a ferrous iron transporter that is important for extracellular and intracellular growth, especially in iron-limited environments. These data represent the first evidence for the importance of ferrous iron transport for intracellular replication by a human pathogen.
Figures




Similar articles
-
lbtA and lbtB are required for production of the Legionella pneumophila siderophore legiobactin.J Bacteriol. 2006 Feb;188(4):1351-63. doi: 10.1128/JB.188.4.1351-1363.2006. J Bacteriol. 2006. PMID: 16452417 Free PMC article.
-
Legionella pneumophila Rhizoferrin Promotes Bacterial Biofilm Formation and Growth within Amoebae and Macrophages.Infect Immun. 2023 Aug 16;91(8):e0007223. doi: 10.1128/iai.00072-23. Epub 2023 Jul 10. Infect Immun. 2023. PMID: 37428036 Free PMC article.
-
Legionella pneumophila type II protein secretion promotes virulence in the A/J mouse model of Legionnaires' disease pneumonia.Infect Immun. 2004 Jan;72(1):310-21. doi: 10.1128/IAI.72.1.310-321.2004. Infect Immun. 2004. PMID: 14688110 Free PMC article.
-
An update on iron acquisition by Legionella pneumophila: new pathways for siderophore uptake and ferric iron reduction.Future Microbiol. 2015;10(5):841-51. doi: 10.2217/fmb.15.21. Future Microbiol. 2015. PMID: 26000653 Free PMC article. Review.
-
Iron acquisition by Legionella pneumophila.Biometals. 2007 Jun;20(3-4):323-31. doi: 10.1007/s10534-006-9057-4. Epub 2006 Dec 16. Biometals. 2007. PMID: 17180462 Review.
Cited by
-
The secreted pyomelanin pigment of Legionella pneumophila confers ferric reductase activity.Infect Immun. 2007 Aug;75(8):4062-70. doi: 10.1128/IAI.00489-07. Epub 2007 Jun 4. Infect Immun. 2007. PMID: 17548481 Free PMC article.
-
Legionella shows a diverse secondary metabolism dependent on a broad spectrum Sfp-type phosphopantetheinyl transferase.PeerJ. 2016 Nov 24;4:e2720. doi: 10.7717/peerj.2720. eCollection 2016. PeerJ. 2016. PMID: 27904811 Free PMC article.
-
The Yfe and Feo transporters are involved in microaerobic growth and virulence of Yersinia pestis in bubonic plague.Infect Immun. 2012 Nov;80(11):3880-91. doi: 10.1128/IAI.00086-12. Epub 2012 Aug 27. Infect Immun. 2012. PMID: 22927049 Free PMC article.
-
Toward a mechanistic understanding of Feo-mediated ferrous iron uptake.Metallomics. 2018 Jul 18;10(7):887-898. doi: 10.1039/c8mt00097b. Metallomics. 2018. PMID: 29953152 Free PMC article. Review.
-
Complete Genome Sequence of Legionella cardiaca Strain H63T, Isolated from a Case of Native Valve Endocarditis.Microbiol Resour Announc. 2023 Jul 18;12(7):e0017523. doi: 10.1128/mra.00175-23. Epub 2023 Jun 13. Microbiol Resour Announc. 2023. PMID: 37310280 Free PMC article.
References
-
- Aisen, P. 1976. Iron metabolism. Ciba Found. Symp. 51: 1-17. - PubMed
-
- Askwith, C., D. Eide, A. Van Ho, P. S. Bernard, L. Li, S. Davis-Kaplan, D. M. Sipe, and J. Kaplan. 1994. The FET3 gene of S. cerevisiae encodes a multicopper oxidase required for ferrous iron uptake. Cell 76:403-410. - PubMed
-
- Bellamy, R. 1999. The natural resistance-associated macrophage protein and susceptibility to intracellular pathogens. Microbes Infect. 1:23-27. - PubMed
-
- Breuer, W., S. Epsztejn, and Z. I. Cabantchnik. 1995. Iron acquired from transferrin by K562 cells is delivered into a cytoplasmic pool of chelatable iron(II). J. Biol. Chem. 270:24209-24215. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

