The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway
- PMID: 12231511
- PMCID: PMC1307617
- DOI: 10.1093/embo-reports/kvf198
The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway
Abstract
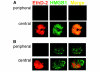
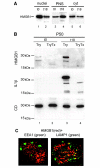
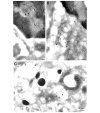
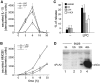
HMGB1, a non-histone nuclear factor, acts extracellularly as a mediator of delayed endotoxin lethality, which raises the question of how a nuclear protein can reach the extracellular space. We show that activation of monocytes results in the redistribution of HMGB1 from the nucleus to cytoplasmic organelles, which display ultrastructural features of endolysosomes. HMGB1 secretion is induced by stimuli triggering lysosome exocytosis. The early mediator of inflammation interleukin (IL)-1beta is also secreted by monocytes through a non-classical pathway involving exocytosis of secretory lysosomes. However, in keeping with their respective role of early and late inflammatory factors, IL-1beta and HMGB1 respond at different times to different stimuli: IL-1beta secretion is induced earlier by ATP, autocrinally released by monocytes soon after activation; HMGB1 secretion is triggered by lysophosphatidylcholine, generated later in the inflammation site. Thus, in monocytes, non-classical secretion can occur through vescicle compartments that are at least partially distinct.
Figures

References
-
- Andrews N.W. (2000) Regulated secretion of conventional lysosomes. Trends Cell Biol., 10, 316–321. - PubMed
-
- Blott E.J. and Griffiths G.M. (2002) Secretory lysosomes. Nat. Rev. Mol. Cell Biol., 3, 122–31. - PubMed
-
- Bodin P. and Burnstock G. (1998) Increased release of ATP from endothelial cells during acute inflammation. Inflamm. Res., 47, 351–354. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

