Combinatorial control of Drosophila eye development by eyeless, homothorax, and teashirt
- PMID: 12231630
- PMCID: PMC187435
- DOI: 10.1101/gad.1009002
Combinatorial control of Drosophila eye development by eyeless, homothorax, and teashirt
Abstract
In Drosophila, the development of the compound eye depends on the movement of a morphogenetic furrow (MF) from the posterior (P) to the anterior (A) of the eye imaginal disc. We define several subdomains along the A-P axis of the eye disc that express distinct combinations of transcription factors. One subdomain, anterior to the MF, expresses two homeobox genes, eyeless (ey) and homothorax (hth), and the zinc-finger gene teashirt (tsh). We provide evidence that this combination of transcription factors may function as a complex and that it plays at least two roles in eye development: it blocks the expression of later-acting transcription factors in the eye development cascade, and it promotes cell proliferation. A key step in the transition from an immature proliferative state to a committed state in eye development is the repression of hth by the BMP-4 homolog Decapentaplegic (Dpp).
Figures
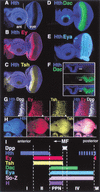
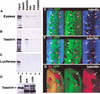
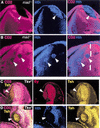

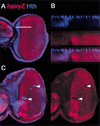




References
-
- Abu-Shaar M, Mann RS. Generation of multiple antagonistic domains along the proximodistal axis during Drosophila leg development. Development. 1998;125:3821–3830. - PubMed
-
- Allen TD, Zhu YX, Hawley TS, Hawley RG. TALE homeoproteins as HOX11-interacting partners in T-cell leukemia. Leuk Lymphoma. 2000;39:241–256. - PubMed
-
- Azpiazu N, Morata G. Function and regulation of homothorax in the wing imaginal disc of Drosophila. Development. 2000;127:2685–2693. - PubMed
-
- Baker NE. Transcription of the segment-polarity gene wingless in the imaginal discs of Drosophila, and the phenotype of a pupal-lethal wg mutation. Development. 1988;102:489–497. - PubMed
-
- ————— Cell proliferation, survival, and death in the Drosophila eye. Semin Cell Dev Biol. 2001;12:499–507. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
