Contribution of a calcium-activated non-specific conductance to NMDA receptor-mediated synaptic potentials in granule cells of the frog olfactory bulb
- PMID: 12231641
- PMCID: PMC2290536
- DOI: 10.1113/jphysiol.2002.024638
Contribution of a calcium-activated non-specific conductance to NMDA receptor-mediated synaptic potentials in granule cells of the frog olfactory bulb
Abstract
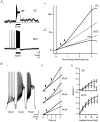
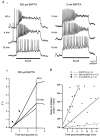
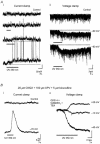
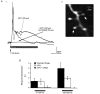
We studied granule cells (GCs) in the intact frog olfactory bulb (OB) by combining whole-cell recordings and functional two-photon Ca(2+) imaging in an in vitro nose-brain preparation. GCs are local interneurones that shape OB output via distributed dendrodendritic inhibition of OB projection neurones, the mitral-tufted cells (MTCs). In contrast to MTCs, GCs exhibited a Ca(2+)-activated non-specific cation conductance (I(CAN)) that could be evoked through strong synaptic stimulation or suprathreshold current injection. Photolysis of the caged Ca(2+) chelator o-nitrophenol-EGTA resulted in activation of an inward current with a reversal potential within the range -20 to +10 mV. I(CAN) in GCs was suppressed by the intracellular Ca(2+) chelator BAPTA (0.5-5.0 mM), but not by EGTA (up to 5 mM). The current persisted in whole-cell recordings for up to 1.5 h post-breakthrough, was observed during perforated-patch recordings and was independent of ionotropic glutamate and GABA(A) receptor activity. In current-clamp mode, GC responses to synaptic stimulation consisted of an initial AMPA-mediated conductance followed by a late-phase APV-sensitive plateau (100-500 ms). BAPTA-mediated suppression of I(CAN) resulted in a selective reduction of the late component of the evoked synaptic potential, consistent with a positive feedback relationship between NMDA receptor (NMDAR) current and I(CAN). I(CAN) requires Ca(2+) influx either through voltage-gated Ca(2+) channels or possibly NMDARs, both of which have a high threshold for activation in GCs, predicting a functional role for this current in the selective enhancement of strong synaptic inputs to GCs.
Figures




Similar articles
-
Functional role of NMDA autoreceptors in olfactory mitral cells.J Neurophysiol. 2000 Jul;84(1):39-50. doi: 10.1152/jn.2000.84.1.39. J Neurophysiol. 2000. PMID: 10899181
-
GABA(B) receptors inhibit dendrodendritic transmission in the rat olfactory bulb.J Neurosci. 2003 Mar 15;23(6):2032-9. doi: 10.1523/JNEUROSCI.23-06-02032.2003. J Neurosci. 2003. PMID: 12657661 Free PMC article.
-
Mechanisms governing dendritic gamma-aminobutyric acid (GABA) release in the rat olfactory bulb.Proc Natl Acad Sci U S A. 2001 Jan 2;98(1):337-42. doi: 10.1073/pnas.98.1.337. Proc Natl Acad Sci U S A. 2001. PMID: 11120892 Free PMC article.
-
SK channel regulation of dendritic excitability and dendrodendritic inhibition in the olfactory bulb.J Neurophysiol. 2005 Dec;94(6):3743-50. doi: 10.1152/jn.00797.2005. Epub 2005 Aug 17. J Neurophysiol. 2005. PMID: 16107526
-
Zinc as a Neuromodulator in the Central Nervous System with a Focus on the Olfactory Bulb.Front Cell Neurosci. 2017 Sep 21;11:297. doi: 10.3389/fncel.2017.00297. eCollection 2017. Front Cell Neurosci. 2017. PMID: 29033788 Free PMC article. Review.
Cited by
-
Calcium buffering in rodent olfactory bulb granule cells and mitral cells.J Physiol. 2009 Sep 15;587(Pt 18):4467-79. doi: 10.1113/jphysiol.2009.174540. Epub 2009 Jul 27. J Physiol. 2009. PMID: 19635818 Free PMC article.
-
Excitatory actions of noradrenaline and metabotropic glutamate receptor activation in granule cells of the accessory olfactory bulb.J Neurophysiol. 2009 Aug;102(2):1103-14. doi: 10.1152/jn.91093.2008. Epub 2009 May 27. J Neurophysiol. 2009. PMID: 19474170 Free PMC article.
-
Ca2+-dependent regulation of a non-selective cation channel from Aplysia bag cell neurones.J Physiol. 2006 Sep 1;575(Pt 2):491-506. doi: 10.1113/jphysiol.2006.105833. Epub 2006 Jun 8. J Physiol. 2006. PMID: 16763004 Free PMC article.
-
Morphological analysis of activity-reduced adult-born neurons in the mouse olfactory bulb.Front Neurosci. 2011 May 9;5:66. doi: 10.3389/fnins.2011.00066. eCollection 2011. Front Neurosci. 2011. PMID: 21602912 Free PMC article.
-
Rapid Feedforward Inhibition and Asynchronous Excitation Regulate Granule Cell Activity in the Mammalian Main Olfactory Bulb.J Neurosci. 2015 Oct 21;35(42):14103-22. doi: 10.1523/JNEUROSCI.0746-15.2015. J Neurosci. 2015. PMID: 26490853 Free PMC article.
References
-
- Arakawa O, Nakahiro M, Narahashi T. Mercury modulation of GABA-activated chloride channels and non-specific cation channels in rat dorsal root ganglion neurons. Brain Research. 1991;551:58–63. - PubMed
-
- Berkowicz DA, Trombley PQ. Dopaminergic modulation at the olfactory nerve synapse. Brain Research. 2000;855:90–99. - PubMed
-
- Chen WR, Xiong W, Shepherd GM. Analysis of relations between NMDA receptors and GABA release at olfactory bulb reciprocal synapses. Neuron. 2000;25:625–633. - PubMed
-
- Delaney KR, Hall BJ. An in vitro preparation of frog nose and brain for the study of odour-evoked oscillatory activity. Journal of Neuroscience Methods. 1996;68:193–202. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

