c-Fos expression in ouabain-treated vascular smooth muscle cells from rat aorta: evidence for an intracellular-sodium-mediated, calcium-independent mechanism
- PMID: 12231642
- PMCID: PMC2290551
- DOI: 10.1113/jphysiol.2002.023259
c-Fos expression in ouabain-treated vascular smooth muscle cells from rat aorta: evidence for an intracellular-sodium-mediated, calcium-independent mechanism
Abstract
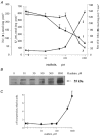
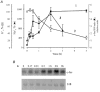
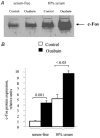
In this study, we examined the effect of Na(+)-K(+) pump inhibition on the expression of early response genes in vascular smooth muscle cells (VSMC) as possible intermediates of the massive RNA synthesis and protection against apoptosis seen in ouabain-treated VSMC in our previous experiments. Incubation of VSMC with ouabain resulted in rapid induction of c-Fos protein expression with an approximately sixfold elevation after 2 h of incubation. c-Jun expression was increased by approximately fourfold after 12 h, whereas expression of activating transcription factor 2, cAMP/Ca(2+) response element binding protein (CREB)-1 and c-Myc was not altered. Markedly augmented c-Fos expression was also observed under Na(+)-K(+) pump inhibition in potassium-depleted medium. Na(+)-K(+) pump inhibition triggered c-Fos expression via elevation of the [Na(+)](i)/[K(+)](i) ratio. This conclusion follows from experiments showing the lack of effect of ouabain on c-Fos expression in high-potassium-low-sodium medium and from the comparison of dose responses of Na(+)-K(+) pump activity, [Na(+)](i) and [K(+)](i) content and c-Fos expression to ouabain. A fourfold increment of c-Fos mRNA was revealed 30 min following addition of ouabain to the incubation medium. At this time point, treatment with ouabain resulted in an approximately fourfold elevation of [Na(+)](i) but did not affect [K(+)](i). Augmented c-Fos expression was also observed under VSMC depolarization in high-potassium medium. Increments in both c-Fos expression and (45)Ca uptake in depolarized VSMC were abolished under inhibition of L-type Ca(2+) channels with 0.1 microM nicardipine. Ouabain did not affect the free [Ca(2+)](i) or the content of exchangeable [Ca(2+)](i). Ouabain-induced c-Fos expression was also insensitive to the presence of nicardipine and [Ca(2+)](o), as well as chelators of [Ca(2+)](o) (EGTA) and [Ca(2+)](i) (BAPTA). The effect of ouabain and serum on c-Fos expression was additive. In contrast to serum, however, ouabain failed to activate the Elk-1, serum response factor, CREB and activator protein-1 transcription factors identified within the c-Fos promoter. These results suggest that Na(+)-K(+) pump inhibition triggers c-Fos expression via [Na(+)](i)-sensitive [Ca(2+)](i)-independent transcription factor(s) distinct from factors interacting with known response elements of this gene promoter.
Figures


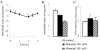



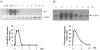
Similar articles
-
Inhibition of Na+,K+ pump affects nucleic acid synthesis and smooth muscle cell proliferation via elevation of the [Na+]i/[K+]i ratio: possible implication in vascular remodelling.J Hypertens. 2001 Sep;19(9):1559-65. doi: 10.1097/00004872-200109000-00007. J Hypertens. 2001. PMID: 11564975
-
Augmented gene expression triggered by Na+,K+-ATPase inhibition: Role of Ca2+i-mediated and -independent excitation-transcription coupling.Cell Calcium. 2017 Dec;68:5-13. doi: 10.1016/j.ceca.2017.10.002. Epub 2017 Oct 9. Cell Calcium. 2017. PMID: 29129208
-
[Na]i -induced c-Fos expression is not mediated by activation of the 5' -promoter containing known transcriptional elements.FEBS J. 2007 Jul;274(14):3557-3567. doi: 10.1111/j.1742-4658.2007.05885.x. Epub 2007 Jun 12. FEBS J. 2007. PMID: 17565602
-
Investigation of factors affecting the intracellular sodium activity in the smooth muscle of guinea-pig ureter.J Physiol. 1987 Apr;385:483-505. doi: 10.1113/jphysiol.1987.sp016503. J Physiol. 1987. PMID: 2443670 Free PMC article. Review.
-
Effect of electrolyte transport on the response of arteriolar smooth muscle.J Cardiovasc Pharmacol. 1984;6 Suppl 1:S82-7. doi: 10.1097/00005344-198400061-00015. J Cardiovasc Pharmacol. 1984. PMID: 6204163 Review.
Cited by
-
Emerging functions of 10 types of TRP cationic channel in vascular smooth muscle.Clin Exp Pharmacol Physiol. 2005 Aug;32(8):597-603. doi: 10.1111/j.1440-1681.2005.04251.x. Clin Exp Pharmacol Physiol. 2005. PMID: 16120184 Free PMC article. Review.
-
Ouabain Induces Transcript Changes and Activation of RhoA/ROCK Signaling in Cultured Epithelial Cells (MDCK).Curr Issues Mol Biol. 2023 Sep 14;45(9):7538-7556. doi: 10.3390/cimb45090475. Curr Issues Mol Biol. 2023. PMID: 37754259 Free PMC article.
-
Ouabain-Induced Cell Death and Survival. Role of α1-Na,K-ATPase-Mediated Signaling and [Na+]i/[K+]i-Dependent Gene Expression.Front Physiol. 2020 Sep 4;11:1060. doi: 10.3389/fphys.2020.01060. eCollection 2020. Front Physiol. 2020. PMID: 33013454 Free PMC article. Review.
-
Ubiquitous [Na+]i/[K+]i-sensitive transcriptome in mammalian cells: evidence for Ca(2+)i-independent excitation-transcription coupling.PLoS One. 2012;7(5):e38032. doi: 10.1371/journal.pone.0038032. Epub 2012 May 29. PLoS One. 2012. PMID: 22666440 Free PMC article.
-
Transcriptomic changes triggered by hypoxia: evidence for HIF-1α-independent, [Na+]i/[K+]i-mediated, excitation-transcription coupling.PLoS One. 2014 Nov 6;9(11):e110597. doi: 10.1371/journal.pone.0110597. eCollection 2014. PLoS One. 2014. PMID: 25375852 Free PMC article.
References
-
- Anderson ME. Connections count. Excitation-contraction meets excitation-transcription coupling. Circulation Research. 2000;86:717–719. - PubMed
-
- Ando K, Omi N, Shimosawa T, Takanashi K, Fujita T. Effect of ouabain on the growth and DNA synthesis of PC12 cells. Journal of Cardiovascular Pharmacology. 2000;37:233–238. - PubMed
-
- Aydemir-Koksoy A, Allen JC. Low concentrations of ouabain induce vascular smooth muscle proliferation. Cellular and Molecular Biology. 2001;47:341–345. - PubMed
-
- Blanco G, Merger RW. Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. American Journal of Physiology. 1998;275:F663–650. - PubMed
-
- Blaustein MP, Lederer WJ. Sodium/calcium exchange: its physiological implications. Physiological Reviews. 1999;79:763–854. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

