The relationship between intracellular [Ca(2+)] and Ca(2+) wave characteristics in permeabilised cardiomyocytes from the rabbit
- PMID: 12231644
- PMCID: PMC2290553
- DOI: 10.1113/jphysiol.2002.021519
The relationship between intracellular [Ca(2+)] and Ca(2+) wave characteristics in permeabilised cardiomyocytes from the rabbit
Abstract
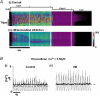
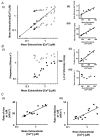
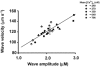
Spontaneous sarcoplasmic reticulum (SR) Ca(2+) release and propagated intracellular Ca(2+) waves are a consequence of cellular Ca(2+) overload in cardiomyocytes. We examined the relationship between average intracellular [Ca(2+)] and Ca(2+) wave characteristics. The amplitude, time course and propagation velocity of Ca(2+) waves were measured using line-scan confocal imaging of beta-escin-permeabilised cardiomyocytes perfused with 10 microM Fluo-3 or Fluo-5F. Spontaneous Ca(2+) waves were evident at cellular [Ca(2+)] > 200 nM. Peak [Ca(2+)] during a wave was 2.0-2.2 microM; the minimum [Ca(2+)] between waves was 120-160 nM; wave frequency was approximately 0.1 Hz. Raising mean cellular [Ca(2+)] caused increases in all three parameters, particularly Ca(2+) wave frequency. Increases in the rate of SR Ca(2+) release and Ca(2+) uptake were observed at higher cellular [Ca(2+)], indicating calcium-sensitive regulation of these processes. At extracellular [Ca(2+)] > 2 microM, the mean [Ca(2+)] inside the permeabilised cell did not increase above 2 microM. This extracellular-intracellular Ca(2+) gradient could be maintained for periods of up to 5 min before the cardiomyocyte developed a sustained and irreversible hypercontraction. Inclusion of mitochondrial inhibitors (2 microM carbonyl cyanide m-chlorophenylhydrazone and 2 microM oligomycin) while perfusing with > 2 microM Ca(2+) abolished the extracellular-intracellular Ca(2+) gradient through the generation of Ca(2+) waves with a higher peak [Ca(2+)] compared to control conditions. Under these conditions, cardiomyocytes rapidly (< 2 min) developed a sustained and irreversible contraction. These results suggest that mitochondrial Ca(2+) uptake acts to delay an increase in [Ca(2+)] by blunting the peak of the Ca(2+) wave.
Figures





References
-
- Cheng H, Lederer MR, Lederer WJ, Cannell MB. Calcium sparks and [Ca2+]i waves in cardiac myocytes. American Journal of Physiology. 1996;270:C148–159. - PubMed
-
- Di Lisa F, Gambassi G, Spurgeon H, Hansford RG. Intramitochondrial free calcium in cardiac myocytes in relation to dehyrogenase activation. Cardiovascular Research. 1993;27:1840–1844. - PubMed
-
- Diaz ME, Trafford AW, O'Neill SC, Eisner DA. A measurable reduction of s. r. Ca content follows spontaneous Ca release in rat ventricular myocytes. Pflügers Archiv. 1997b;434:852–854. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

