A push-pull approach to maximize vaccine efficacy: abrogating suppression with an IL-13 inhibitor while augmenting help with granulocyte/macrophage colony-stimulating factor and CD40L
- PMID: 12232042
- PMCID: PMC130579
- DOI: 10.1073/pnas.192251199
A push-pull approach to maximize vaccine efficacy: abrogating suppression with an IL-13 inhibitor while augmenting help with granulocyte/macrophage colony-stimulating factor and CD40L
Abstract
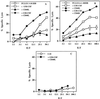
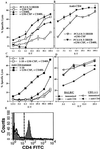
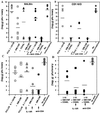
Although a role for CD4(+) helper cells in CD8(+) cytotoxic T lymphocyte (CTL) induction by vaccines is widely recognized, much less is known about a counterbalancing role of CD4(+) T cells in down-modulating this response, or about ways to optimize vaccine responses through abrogation of this negative regulatory mechanism. Here, we discovered a synergistic enhancement of vaccine-mediated CTL induction and protection by the relief of suppression through depletion of regulatory CD4(+) cells, including CD4(+) NKT cells, or blockade of IL-13 made by these cells, combined with the cytokine granulocyte/macrophage colony-stimulating factor and the costimulatory molecule CD40L. Indeed, in the absence of helper epitopes, granulocyte/macrophage colony-stimulating factor and the helper-mimetic molecule CD40L are not sufficient to replace help to induce CTL without abrogation of CD4(+) T cell-mediated suppression, suggesting a role for T cell help in overcoming suppression. The increased CTL induction translated to striking protection against viral infection by a vaccine by using this synergistic combined approach. These results argue for a push-pull approach to maximize vaccine efficacy, especially for HIV and cancer.
Figures

References
-
- Rowland-Jones S, Tan R, McMichael A. Adv Immunol. 1997;65:277–346. - PubMed
-
- Goulder P J R, Rowland-Jones S L, McMichael A J, Walker B D. AIDS. 1999;13:S121–S136. - PubMed
-
- Oscherwitz J, Gotch F M, Cease K B, Berzofsky J A. AIDS. 1999;13:S163–S174. - PubMed
-
- Schmitz J E, Kuroda M J, Santra S, Sasseville V G, Simon M A, Lifton M A, Racz P, Tenner-Racz K, Dalesandro M, Scallon B J, et al. Science. 1999;283:857–860. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

