Evaluation of 2-deoxy-D-glucose as a chemotherapeutic agent: mechanism of cell death
- PMID: 12232767
- PMCID: PMC2364258
- DOI: 10.1038/sj.bjc.6600547
Evaluation of 2-deoxy-D-glucose as a chemotherapeutic agent: mechanism of cell death
Abstract
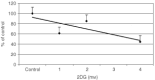
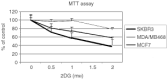
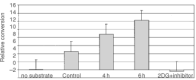
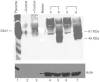
Nutrient deprivation has been shown to cause cancer cell death. To exploit nutrient deprivation as anti-cancer therapy, we investigated the effects of the anti-metabolite 2-deoxy-D-glucose on breast cancer cells in vitro. This compound has been shown to inhibit glucose metabolism. Treatment of human breast cancer cell lines with 2-deoxy-D-glucose results in cessation of cell growth in a dose dependent manner. Cell viability as measured by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide conversion assay and clonogenic survival are decreased with 2-deoxy-D-glucose treatment indicating that 2-deoxy-D-glucose causes breast cancer cell death. The cell death induced by 2-deoxy-D-glucose was found to be due to apoptosis as demonstrated by induction of caspase 3 activity and cleavage of poly (ADP-ribose) polymerase. Breast cancer cells treated with 2-deoxy-D-glucose express higher levels of Glut1 transporter protein as measured by Western blot analysis and have increased glucose uptake compared to non-treated breast cancer cells. From these results we conclude that 2-deoxy-D-glucose treatment causes death in human breast cancer cell lines by the activation of the apoptotic pathway. Our data suggest that breast cancer cells treated with 2-deoxy-D-glucose accelerate their own demise by initially expressing high levels of glucose transporter protein, which allows increased uptake of 2-deoxy-D-glucose, and subsequent induction of cell death. These data support the targeting of glucose metabolism as a site for chemotherapeutic intervention by agents such as 2-deoxy-D-glucose.
Figures



References
-
- AlojLCaracoCJagodaEEckelmanWNeumannR1999Glut-1 and hexokinase expression: relationship with 2-fluoro-2-deoxy-D-glucose uptake in A431 and T47D cells in culture Cancer Res 5947094714 - PubMed
-
- AmundsonSMyersTScudieroDKitadaSReedJFornaceA2000An informatics approach identifying markers of chemosensitivity in human cancer cell lines Cancer Res 6061016110 - PubMed
-
- ArdehaliHPrintzRWhitesellRMayJGrannerD1999Functional interaction between the N- and C-terminal halves of human hexokinase II J Biol Chem 2741598615989 - PubMed
-
- ArdehaliHYanoHPrintzRKochSWhitesellRMayJGrannerD1996Functional organization of mammalian hexokinase II J Biol Chem 27118491852 - PubMed
-
- AroraKParryDPedersenP1992Hexokinase receptors: preferential enzyme binding in normal cells to non-mitochondrial sites and in transformed cells to mitochondrial sites J Bioenergetics Biomembranes 244753 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

