The role of aquaporins in root water uptake
- PMID: 12234142
- PMCID: PMC4240399
- DOI: 10.1093/aob/mcf199
The role of aquaporins in root water uptake
Abstract
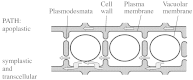
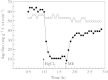
The capacity of roots to take up water is determined in part by the resistance of living tissues to radial water flow. Both the apoplastic and cell-to-cell paths mediate water transport in these tissues but the contribution of cell membranes to the latter path has long been difficult to estimate. Aquaporins are water channel proteins that are expressed in various membrane compartments of plant cells, including the plasma and vacuolar membranes. Plant aquaporins are encoded by a large multigene family, with 35 members in Arabidopsis thaliana, and many of these aquaporins show a cell-specific expression pattern in the root. Mercury acts as an efficient blocker of most aquaporins and has been used to demonstrate the significant contribution of water channels to overall root water transport. Aquaporin-rich membranes may be needed to facilitate intense water flow across root tissues and may represent critical points where an efficient and spatially restricted control of water uptake can be exerted. Roots, in particular, show a remarkable capacity to alter their water permeability over the short term (i.e. in a few hours to less than 2-3 d) in response to many stimuli, such as day/night cycles, nutrient deficiency or stress. Recent data suggest that these rapid changes can be mostly accounted for by changes in cell membrane permeability and are mediated by aquaporins. Although the processes that allow perception of environmental changes by root cells and subsequent aquaporin regulation are nearly unknown, the study of root aquaporins provides an interesting model to understand the regulation of water transport in plants and sheds light on the basic mechanisms of water uptake by roots.
Figures


References
-
- AgreP, Bonhivers M, Borgnia MJ.1998. The aquaporins, blueprints for cellular plumbing systems. Journal of Biological Chemistry 273: 14659–14662. - PubMed
-
- AmodeoG, Dorr R, Vallejo A, Sutka M, Parisi M.1999. Radial and axial water transport in the sugar beet storage root. Journal of Experimental Botany 50: 509–516.
-
- BarrieuF, Morillon R, Chrispeels M.2000. Modulation of aquaporin gene expression in Arabidopsis leads to altered membrane water permeability. In: Hohmann S, Nielsen S, eds. Molecular biology and physiology of water and solute transport New York, USA: Kluwer Academic/Plenum Publishers, 255–259.

