From the cell biology to the development of new chemotherapeutic approaches against trypanosomatids: dreams and reality
- PMID: 12234386
- PMCID: PMC119324
- DOI: 10.1186/1475-9292-1-3
From the cell biology to the development of new chemotherapeutic approaches against trypanosomatids: dreams and reality
Abstract
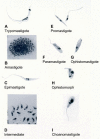
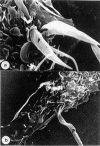
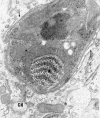
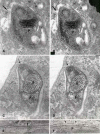
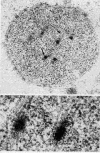
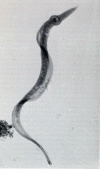
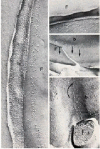
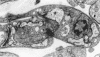
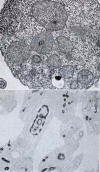
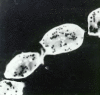
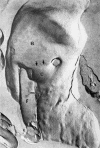
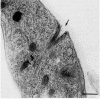
Members of the Trypanosomatidae family comprise a large number of species that are causative agents of important diseases such as sleeping sickness, Chagas' disease and Leishmaniasis. These organisms are also of biological interest since they are able to change the morphology according to the environment where they live, through a process of reversible cell transformation, and possess structures and organelles that are not found in mammalian cells. This review analyses the process of transformation, which takes place during the life cycle of Trypanosoma cruzi in the vertebrate and invertebrate hosts. Special attention is given to the interaction of the parasite with vertebrate cells. In addition, the present knowledge of structures and organelles such as the nucleus, the plasma membrane, the sub-pellicular microtubules, the flagellum, the kinetoplast-mitochondrion complex, the peroxisome (glycosome), the acidocalcisome and the structures and organelles involved in the endocytic pathway, is reviewed from a cell biology perspective. The possible use of available data for the development of new anti parasite drugs is also discussed.
Figures










References
-
- Briones MRS, Souto RP, Stolf BS, Zingales B. The evolution of two Trypanosoma cruzi subgroups inferred from rRNA genes can be correlated with the interchange of American mammalian faunas in the Cenozoic and has implications to pathogenicity and host specificity. Mol Biochem Parasitol. 1999;104:219–232. doi: 10.1016/S0166-6851(99)00155-3. - DOI - PubMed
-
- Carvalho TU, De Souza W. Infectivity of amastigotes of Trypanosoma cruzi. Rev Inst Med Trop São Paulo. 1986;28:205–212. - PubMed
-
- Meyer H, De Oliveira M. Cultivation of Trypanosoma cruzi in tissue cultures: a four year study. Parasitology. 1948;39:91–94. - PubMed
LinkOut - more resources
Full Text Sources

