Sprouty2 attenuates epidermal growth factor receptor ubiquitylation and endocytosis, and consequently enhances Ras/ERK signalling
- PMID: 12234920
- PMCID: PMC126289
- DOI: 10.1093/emboj/cdf493
Sprouty2 attenuates epidermal growth factor receptor ubiquitylation and endocytosis, and consequently enhances Ras/ERK signalling
Abstract
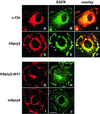
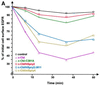
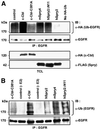
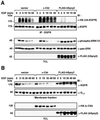
Drosophila Sprouty (dSpry) was genetically identified as a novel antagonist of fibroblast growth factor receptor (FGFR), epidermal growth factor receptor (EGFR) and Sevenless signalling, ostensibly by eliciting its response on the Ras/MAPK pathway. Four mammalian sprouty genes have been cloned, which appear to play an inhibitory role mainly in FGF- mediated lung and limb morphogenesis. Evidence is presented herein that describes the functional implications of the direct association between human Sprouty2 (hSpry2) and c-Cbl, and its impact on the cellular localization and signalling capacity of EGFR. Contrary to the consensus view that Spry2 is a general inhibitor of receptor tyrosine kinase signalling, hSpry2 was shown to abrogate EGFR ubiquitylation and endocytosis, and sustain EGF-induced ERK signalling that culminates in differentiation of PC12 cells. Correlative evidence showed the failure of hSpry2DeltaN11 and mSpry4, both deficient in c-Cbl binding, to instigate these effects. hSpry2 interacts specifically with the c-Cbl RING finger domain and displaces UbcH7 from its binding site on the E3 ligase. We conclude that hSpry2 potentiates EGFR signalling by specifically intercepting c-Cbl-mediated effects on receptor down-regulation.
Figures




Similar articles
-
Evidence for direct interaction between Sprouty and Cbl.J Biol Chem. 2001 Feb 23;276(8):5866-75. doi: 10.1074/jbc.M006945200. Epub 2000 Oct 26. J Biol Chem. 2001. PMID: 11053437
-
Tyrosine phosphorylation of Sprouty2 enhances its interaction with c-Cbl and is crucial for its function.J Biol Chem. 2003 Aug 29;278(35):33456-64. doi: 10.1074/jbc.M301317200. Epub 2003 Jun 18. J Biol Chem. 2003. PMID: 12815057
-
hSpry2 is targeted to the ubiquitin-dependent proteasome pathway by c-Cbl.Curr Biol. 2003 Feb 18;13(4):308-14. doi: 10.1016/s0960-9822(03)00086-1. Curr Biol. 2003. PMID: 12593796
-
Sprouty: how does the branch manager work?J Cell Sci. 2003 Aug 1;116(Pt 15):3061-8. doi: 10.1242/jcs.00652. J Cell Sci. 2003. PMID: 12829736 Review.
-
Negative regulation of receptor tyrosine kinases: unexpected links to c-Cbl and receptor ubiquitylation.Cell Res. 2005 Jan;15(1):66-71. doi: 10.1038/sj.cr.7290268. Cell Res. 2005. PMID: 15686631 Review.
Cited by
-
K63-linked ubiquitination of DYRK1A by TRAF2 alleviates Sprouty 2-mediated degradation of EGFR.Cell Death Dis. 2021 Jun 11;12(6):608. doi: 10.1038/s41419-021-03887-2. Cell Death Dis. 2021. PMID: 34117217 Free PMC article.
-
HECT domain-containing E3 ubiquitin ligase Nedd4 interacts with and ubiquitinates Sprouty2.J Biol Chem. 2010 Jan 1;285(1):255-64. doi: 10.1074/jbc.M109.030882. Epub 2009 Oct 28. J Biol Chem. 2010. PMID: 19864419 Free PMC article.
-
Feasibility of using gene expression analysis to study canine soft tissue sarcomas.Mamm Genome. 2010 Dec;21(11-12):577-82. doi: 10.1007/s00335-010-9298-y. Epub 2010 Nov 13. Mamm Genome. 2010. PMID: 21076837 Free PMC article.
-
Inhibition of DYRK1A-EGFR axis by p53-MDM2 cascade mediates the induction of cellular senescence.Cell Death Dis. 2019 Mar 25;10(4):282. doi: 10.1038/s41419-019-1521-5. Cell Death Dis. 2019. PMID: 30910997 Free PMC article.
-
Mechanisms for Regulating and Organizing Receptor Signaling by Endocytosis.Annu Rev Biochem. 2021 Jun 20;90:709-737. doi: 10.1146/annurev-biochem-081820-092427. Epub 2021 Feb 19. Annu Rev Biochem. 2021. PMID: 33606955 Free PMC article. Review.
References
-
- Ardley H.C., Tan,N.G., Rose,S.A., Markham,A.F. and Robinson,P.A. (2001) Features of the Parkin/Ariadne ubiquitin ligase, HHARI, which regulates its interaction with the ubiquitin-conjugating enzyme, UbcH7. J. Biol. Chem., 276, 19640–19647. - PubMed
-
- Bao J., Alroy,I., Waterman,H., Schejter,E.D., Brodie,C., Gruenberg,J. and Yarden,Y. (2000) Threonine phosphorylation diverts internalized epidermal growth factor receptors from a degradative pathway to the recycling endosome. J. Biol. Chem., 275, 26178–26186. - PubMed
-
- Bartkiewicz M., Houghton,A. and Baron,R. (1999) Leucine zipper-mediated homo-dimerization of the adaptor protein c-Cbl. A role in c-Cbl’s tyrosine phosphorylation and its association with epidermal growth factor receptor. J. Biol. Chem., 274, 30887–30895. - PubMed
-
- Basu S.K., Goldstein,J.L., Anderson,R.G. and Brown,M.S. (1981) Monensin interrupts the recycling of low density lipoprotein receptors in human fibroblasts. Cell, 24, 493–502. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

