Pleiotropic defects in TCR signaling in a Vav-1-null Jurkat T-cell line
- PMID: 12234921
- PMCID: PMC126295
- DOI: 10.1093/emboj/cdf499
Pleiotropic defects in TCR signaling in a Vav-1-null Jurkat T-cell line
Abstract
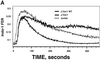
The Rac/Rho-specific guanine nucleotide exchange factor, Vav-1, is a key component of the T-cell antigen receptor (TCR)-linked signaling machinery. Here we have used somatic cell gene-targeting technology to generate a Vav-1-deficient Jurkat T-cell line. The J.Vav1 cell line exhibits dramatic defects in TCR-dependent interleukin (IL)-2 promoter activation, accompanied by significant reductions in the activities of the NFAT(IL-2), NFkappaB, AP-1 and REAP transcription factors that bind to the IL-2 promoter region. In contrast, loss of Vav-1 had variable effects on early TCR-stimulated signaling events. J.Vav1 cells display a selective defect in sustained Ca(2+) signaling during TCR stimulation, and complementation of this abnormality by exogenously introduced Vav-1 is dependent on the Vav-1 calponin homology domain. While JNK activation was severely impaired, the stimulation of Ras, ERK and protein kinase C-theta activities, as well as the mobilization of lipid rafts, appeared normal in the J.Vav1 cells. Finally, evidence is presented to suggest that the alternative Vav family members, Vav-2 and Vav-3, are activated during TCR ligation, and partially compensate for the loss of Vav-1 in Jurkat T cells.
Figures














References
-
- Abraham R.T. (2000) Mutant T cell lines as model systems for the dissection of T cell antigen receptor signaling pathways. Immunol. Res., 22, 95–117. - PubMed
-
- Astoul E., Edmunds,C., Cantrell,D.A. and Ward,S.G. (2001) Pl 3-K and T-cell activation: limitations of T-leukemic cell lines as signaling models. Trends Immunol., 22, 490–496. - PubMed
-
- Bi K., Tanaka,Y., Coudronniere,N., Sugie,K., Hong,S.J., van Stipdonk, M.J.B. and Altman,A. (2001) Antigen-induced translocation of PKC-θ to membrane rafts is required for T cell activation. Nat. Immunol., 2, 556–563. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

