Fission yeast Mor2/Cps12, a protein similar to Drosophila Furry, is essential for cell morphogenesis and its mutation induces Wee1-dependent G(2) delay
- PMID: 12234926
- PMCID: PMC126291
- DOI: 10.1093/emboj/cdf495
Fission yeast Mor2/Cps12, a protein similar to Drosophila Furry, is essential for cell morphogenesis and its mutation induces Wee1-dependent G(2) delay
Abstract
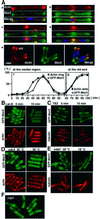
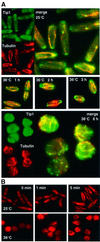
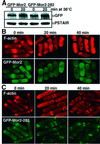
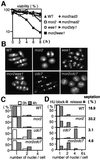
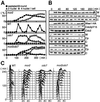
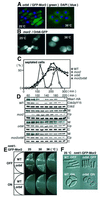
Fission yeast cells identify growing regions at the opposite ends of the cell, producing the rod-like shape. The positioning of the growth zone(s) and the polarized growth require CLIP170-like protein Tip1 and the Ndr kinase Orb6, respectively. Here, we show that the mor2/cps12 mutation disrupts the localization of F-actin at the cell ends, producing spherical cells and concomitantly inducing a G(2) delay at 36 degrees C. Mor2 is important for the localization of F-actin at the cell end(s) but not at the medial region, and is essential for the restriction of the growth zone(s) where Tip1 targets. Mor2 is homologous to the Drosophila Furry protein, which is required to maintain the integrity of cellular extensions, and is localized at both cell ends and the medial region of the cell in an actin-dependent fashion. Cellular localization of Mor2 and Orb6 was interdependent. The tyrosine kinase Wee1 is necessary for the G(2) delay and maintenance of viability of the mor2 mutant. These results indicate that Mor2 plays an essential role in cell morphogenesis in concert with Orb6, and the mutation activates the mechanism coordinating morphogenesis with cell cycle progression.
Figures


References
-
- Alfa C., Fantes,P., Hyams,J., McLeod,M. and Warbrick,E. (1993) Experiments with Fission Yeast: a Laboratory Course Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
-
- Aligue R., Wu,L. and Russell,P. (1997) Regulation of Schizosaccharomyces pombe Wee1 tyrosine kinase. J. Biol. Chem., 272, 13320–13325. - PubMed
-
- Bahler J., Wu,J., Longtine,M.S., Shah,N.G., McKenzie,A.III, Steever,A.B., Wach,A., Philippsen,P. and Pringle,J.R. (1998) Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast, 14, 943–951. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

