Dual mode of degradation of Cdc25 A phosphatase
- PMID: 12234927
- PMCID: PMC126287
- DOI: 10.1093/emboj/cdf491
Dual mode of degradation of Cdc25 A phosphatase
Abstract
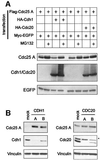
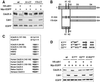
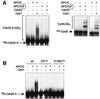
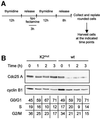
The Cdc25 dual-specificity phosphatases control progression through the eukaryotic cell division cycle by activating cyclin-dependent kinases. Cdc25 A regulates entry into S-phase by dephosphorylating Cdk2, it cooperates with activated oncogenes in inducing transformation and is overexpressed in several human tumors. DNA damage or DNA replication blocks induce phosphorylation of Cdc25 A and its subsequent degradation via the ubiquitin-proteasome pathway. Here we have investigated the regulation of Cdc25 A in the cell cycle. We found that Cdc25 A degradation during mitotic exit and in early G(1) is mediated by the anaphase-promoting complex or cyclosome (APC/C)(Cdh1) ligase, and that a KEN-box motif in the N-terminus of the protein is required for its targeted degradation. Interestingly, the KEN-box mutated protein remains unstable in interphase and upon ionizing radiation exposure. Moreover, SCF (Skp1/Cullin/F-box) inactivation using an interfering Cul1 mutant accumulates and stabilizes Cdc25 A. The presence of Cul1 and Skp1 in Cdc25 A immunocomplexes suggests a direct involvement of SCF in Cdc25 A degradation during interphase. We propose that a dual mechanism of regulated degradation allows for fine tuning of Cdc25 A abundance in response to cell environment.
Figures



Similar articles
-
Hsl1p, a Swe1p inhibitor, is degraded via the anaphase-promoting complex.Mol Cell Biol. 2000 Jul;20(13):4614-25. doi: 10.1128/MCB.20.13.4614-4625.2000. Mol Cell Biol. 2000. PMID: 10848588 Free PMC article.
-
D box and KEN box motifs in budding yeast Hsl1p are required for APC-mediated degradation and direct binding to Cdc20p and Cdh1p.Genes Dev. 2001 Sep 15;15(18):2381-95. doi: 10.1101/gad.917901. Genes Dev. 2001. PMID: 11562348 Free PMC article.
-
Timing of APC/C substrate degradation is determined by fzy/fzr specificity of destruction boxes.EMBO J. 2002 Sep 2;21(17):4500-10. doi: 10.1093/emboj/cdf452. EMBO J. 2002. PMID: 12198152 Free PMC article.
-
Structure, function and mechanism of the anaphase promoting complex (APC/C).Q Rev Biophys. 2011 May;44(2):153-90. doi: 10.1017/S0033583510000259. Epub 2010 Nov 22. Q Rev Biophys. 2011. PMID: 21092369 Review.
-
SCF and APC: the Yin and Yang of cell cycle regulated proteolysis.Curr Opin Cell Biol. 1998 Dec;10(6):759-68. doi: 10.1016/s0955-0674(98)80119-1. Curr Opin Cell Biol. 1998. PMID: 9914180 Review.
Cited by
-
APC/C(Cdh1)-mediated degradation of the F-box protein NIPA is regulated by its association with Skp1.PLoS One. 2011;6(12):e28998. doi: 10.1371/journal.pone.0028998. Epub 2011 Dec 20. PLoS One. 2011. PMID: 22205987 Free PMC article.
-
Loss of cyclin-dependent kinase 2 (CDK2) inhibitory phosphorylation in a CDK2AF knock-in mouse causes misregulation of DNA replication and centrosome duplication.Mol Cell Biol. 2012 Apr;32(8):1421-32. doi: 10.1128/MCB.06721-11. Epub 2012 Feb 13. Mol Cell Biol. 2012. PMID: 22331465 Free PMC article.
-
Pro-apoptotic role of Cdc25A: activation of cyclin B1/Cdc2 by the Cdc25A C-terminal domain.J Biol Chem. 2010 Jun 4;285(23):17833-45. doi: 10.1074/jbc.M109.078386. Epub 2010 Apr 5. J Biol Chem. 2010. PMID: 20368335 Free PMC article.
-
Fluorescence fluctuation analysis reveals PpV dependent Cdc25 protein dynamics in living embryos.PLoS Genet. 2020 Apr 6;16(4):e1008735. doi: 10.1371/journal.pgen.1008735. eCollection 2020 Apr. PLoS Genet. 2020. PMID: 32251417 Free PMC article.
-
CDC25A phosphatase controls meiosis I progression in mouse oocytes.Dev Biol. 2008 May 1;317(1):260-9. doi: 10.1016/j.ydbio.2008.02.028. Epub 2008 Mar 4. Dev Biol. 2008. PMID: 18367163 Free PMC article.
References
-
- Carrano A.C., Eytan,E., Hershko,A. and Pagano,M. (1999) SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat. Cell Biol., 1, 193–199. - PubMed
-
- Cenciarelli C., Chiaur,D.S., Guardavaccaro,D., Parks,W., Vidal,M. and Pagano,M. (1999) Identification of a family of human F-box proteins. Curr. Biol., 9, 1177–1179. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

