Synergistic enhancement of bone formation and healing by stem cell-expressed VEGF and bone morphogenetic protein-4
- PMID: 12235106
- PMCID: PMC151123
- DOI: 10.1172/JCI15153
Synergistic enhancement of bone formation and healing by stem cell-expressed VEGF and bone morphogenetic protein-4
Abstract
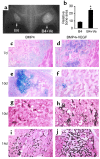
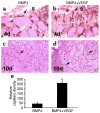
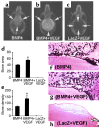
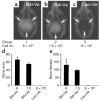
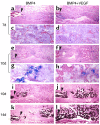
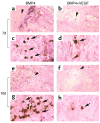
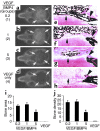
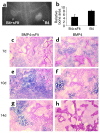
We investigated the interaction between angiogenic and osteogenic factors in bone formation and bone healing with ex vivo gene therapy using muscle-derived stem cells genetically engineered to express human bone morphogenetic protein-4 (BMP4), VEGF, or VEGF-specific antagonist (soluble Flt1). Our results show that although VEGF alone did not improve bone regeneration, it acted synergistically with BMP4 to increase recruitment of mesenchymal stem cells, to enhance cell survival, and to augment cartilage formation in the early stages of endochondral bone formation. These early effects, coupled with accelerated cartilage resorption, eventually led to a significant enhancement of bone formation and bone healing. The beneficial effect of VEGF on bone healing elicited by BMP4 depends critically on the ratio of VEGF to BMP4, with an improper ratio leading to detrimental effects on bone healing. Finally, we show that soluble Flt1 inhibits bone formation elicited by BMP4. Thus, VEGF plays an important role in bone formation elicited by BMP4, and it can significantly enhance BMP4-elicited bone formation and regeneration through multiple mechanisms. This study has important implications for the formulation of new strategies to improve bone healing through increasing mesenchymal stem cell recruitment and survival, in combination with muscle-derived stem cell-based gene therapy.
Figures
References
-
- Praemer, A., Furner, S., and Rice, D.P. 1992. Musculoskeletal conditions in the United States. Proceedings of the American Academy of Orthopaedic Surgeons. Park Ridge, Illinois, USA. 83–124.
-
- Urist MR. Bone formation by autoinduction. Science. 1965;150:893–899. - PubMed
-
- Wozney JM, et al. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528–1534. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

