TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity
- PMID: 12235115
- PMCID: PMC151130
- DOI: 10.1172/JCI15606
TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity
Abstract
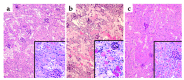
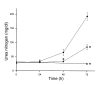
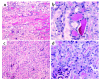
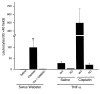
The purpose of these studies was to examine the role of cytokines in the pathogenesis of cisplatin nephrotoxicity. Injection of mice with cisplatin (20 mg/kg) led to severe renal failure. The expression of cytokines, chemokines, and ICAM-1 in kidney was measured by ribonuclease protection assays and RT-PCR. We found significant upregulation of TNF-alpha, TGF-beta, RANTES, MIP-2, MCP-1, TCA3, IL-1beta, and ICAM-1 in kidneys from cisplatin-treated animals. In addition, serum, kidney, and urine levels of TNF-alpha measured by ELISA were increased by cisplatin. Inhibitors of TNF-alpha production (GM6001, pentoxifylline) and TNF-alpha Ab's reduced serum and kidney TNF-alpha protein levels and also blunted the cisplatin-induced increases in TNF-alpha, TGF-beta, RANTES, MIP-2, MCP-1, and IL-1beta, but not ICAM-1, mRNA. In addition, the TNF-alpha inhibitors also ameliorated cisplatin-induced renal dysfunction and reduced cisplatin-induced structural damage. Likewise, TNF-alpha-deficient mice were resistant to cisplatin nephrotoxicity. These results indicate cisplatin nephrotoxicity is characterized by activation of proinflammatory cytokines and chemokines. TNF-alpha appears to play a central role in the activation of this cytokine response and also in the pathogenesis of cisplatin renal injury.
Figures
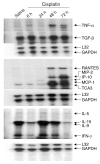
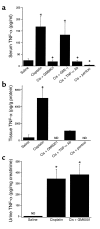

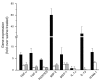
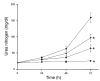
Comment in
-
Cancer therapy and renal injury.J Clin Invest. 2002 Sep;110(6):743-5. doi: 10.1172/JCI16568. J Clin Invest. 2002. PMID: 12235103 Free PMC article. No abstract available.
References
-
- Lebwohl D, Canetta R. Clinical development of platinum complexes in cancer therapy: an historical perspective and an update. Eur J Cancer. 1998;34:1522–1534. - PubMed
-
- Ries F, Klastersky J. Nephrotoxicity induced by cancer chemotherapy with special emphasis on cisplatin toxicity. Am J Kidney Dis. 1986;8:368–379. - PubMed
-
- Leibbrandt ME, Wolfgang GH, Metz AL, Ozobia AA, Haskins JR. Critical subcellular targets of cisplatin and related platinum analogs in rat renal proximal tubule cells. Kidney Int. 1995;48:761–770. - PubMed
-
- Sugiyama S, et al. Adverse effects of anti-tumor drug, cisplatin, on rat kidney mitochrondria: disturbances in glutathione peroxidase activity. Biochem Biophys Res Commun. 1989;159:1121–1127. - PubMed
-
- Matsushima H, Yonemura K, Ohishi K, Hishida A. The role of oxygen free radicals in cisplatin-induced acute renal failure in rats. J Lab Clin Med. 1998;131:518–526. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

