Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets
- PMID: 12235117
- PMCID: PMC151125
- DOI: 10.1172/JCI15318
Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets
Erratum in
-
Glucose-induced β cell production of IL-1β contributes to glucotoxicity in human pancreatic islets.J Clin Invest. 2017 Apr 3;127(4):1589. doi: 10.1172/JCI92172. Epub 2017 Apr 3. J Clin Invest. 2017. PMID: 28368291 Free PMC article. No abstract available.
Abstract
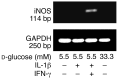
In type 2 diabetes, chronic hyperglycemia is suggested to be detrimental to pancreatic beta cells, causing impaired insulin secretion. IL-1beta is a proinflammatory cytokine acting during the autoimmune process of type 1 diabetes. IL-1beta inhibits beta cell function and promotes Fas-triggered apoptosis in part by activating the transcription factor NF-kappaB. Recently, we have shown that increased glucose concentrations also induce Fas expression and beta cell apoptosis in human islets. The aim of the present study was to test the hypothesis that IL-1beta may mediate the deleterious effects of high glucose on human beta cells. In vitro exposure of islets from nondiabetic organ donors to high glucose levels resulted in increased production and release of IL-1beta, followed by NF-kappaB activation, Fas upregulation, DNA fragmentation, and impaired beta cell function. The IL-1 receptor antagonist protected cultured human islets from these deleterious effects. beta cells themselves were identified as the islet cellular source of glucose-induced IL-1beta. In vivo, IL-1beta-producing beta cells were observed in pancreatic sections of type 2 diabetic patients but not in nondiabetic control subjects. Similarly, IL-1beta was induced in beta cells of the gerbil Psammomys obesus during development of diabetes. Treatment of the animals with phlorizin normalized plasma glucose and prevented beta cell expression of IL-1beta. These findings implicate an inflammatory process in the pathogenesis of glucotoxicity in type 2 diabetes and identify the IL-1beta/NF-kappaB pathway as a target to preserve beta cell mass and function in this condition.
Figures





References
-
- Unger RH, Grundy S. Hyperglycaemia as an inducer as well as a consequence of impaired islet cell function and insulin resistance: implications for the management of diabetes. Diabetologia. 1985;28:119–121. - PubMed
-
- Kaiser N, Corcos AP, Sarel I, Cerasi E. Monolayer culture of adult rat pancreatic islets on extracellular matrix: modulation of B-cell function by chronic exposure to high glucose. Endocrinology. 1991;129:2067–2076. - PubMed
-
- Robertson RP. Type II diabetes, glucose “non-sense,” and islet desensitization. Diabetes. 1989;38:1501–1505. - PubMed
-
- Rossetti L, Giaccari A, DeFronzo RA. Glucose toxicity. Diabetes Care. 1990;13:610–630. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

