Involvement of TRPC in the abnormal calcium influx observed in dystrophic (mdx) mouse skeletal muscle fibers
- PMID: 12235126
- PMCID: PMC2173225
- DOI: 10.1083/jcb.200203091
Involvement of TRPC in the abnormal calcium influx observed in dystrophic (mdx) mouse skeletal muscle fibers
Abstract
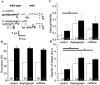
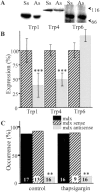
Duchenne muscular dystrophy results from the lack of dystrophin, a cytoskeletal protein associated with the inner surface membrane, in skeletal muscle. The absence of dystrophin induces an abnormal increase of sarcolemmal calcium influx through cationic channels in adult skeletal muscle fibers from dystrophic (mdx) mice. We observed that the activity of these channels was increased after depletion of the stores of calcium with thapsigargin or caffeine. By analogy with the situation observed in nonexcitable cells, we therefore hypothesized that these store-operated channels could belong to the transient receptor potential channel (TRPC) family. We measured the expression of TRPC isoforms in normal and mdx adult skeletal muscles fibers, and among the seven known isoforms, five were detected (TRPC1, 2, 3, 4, and 6) by RT-PCR. Western blot analysis and immunocytochemistry of normal and mdx muscle fibers demonstrated the localization of TRPC1, 4, and 6 proteins at the plasma membrane. Therefore, an antisense strategy was used to repress these TRPC isoforms. In parallel with the repression of the TRPCs, we observed that the occurrence of calcium leak channels was decreased to one tenth of its control value (patch-clamp technique), showing the involvement of TRPC in the abnormal calcium influx observed in dystrophic fibers.
Figures
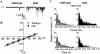




References
-
- Bertorini, T.E., S.K. Bhattacharya, G.M. Palmieri, C.M. Chesney, D. Pifer, and B. Baker. 1982. Muscle calcium and magnesium content in Duchenne muscular dystrophy. Neurology. 32:1088–1092. - PubMed
-
- Boulay, G., D.M. Brown, N. Qin, M. Jiang, A. Dietrich, M.X. Zhu, Z. Chen, M. Birnbaumer, K. Mikoshiba, and L. Birnbaumer. 1999. Modulation of Ca2+ entry by polypeptides of the inositol 1,4, 5-trisphosphate receptor (IP3R) that bind transient receptor potential (TRP): evidence for roles of TRP and IP3R in store depletion-activated Ca2+ entry. Proc. Natl. Acad. Sci. USA. 96:14955–14960. - PMC - PubMed
-
- Clapham, D.E., L.W. Runnels, and C. Strubing. 2001. The TRP ion channel family. Nat. Rev. Neurosci. 2:387–396. - PubMed

