A crucial role for the p110delta subunit of phosphatidylinositol 3-kinase in B cell development and activation
- PMID: 12235209
- PMCID: PMC2194055
- DOI: 10.1084/jem.20020805
A crucial role for the p110delta subunit of phosphatidylinositol 3-kinase in B cell development and activation
Abstract
Mice lacking the p110delta catalytic subunit of phosphatidylinositol 3-kinase have reduced numbers of B1 and marginal zone B cells, reduced levels of serum immunoglobulins, respond poorly to immunization with type II thymus-independent antigen, and are defective in their primary and secondary responses to thymus-dependent antigen. p110delta(-/-) B cells proliferate poorly in response to B cell receptor (BCR) or CD40 signals in vitro, fail to activate protein kinase B, and are prone to apoptosis. p110delta function is required for BCR-mediated calcium flux, activation of phosphlipaseCgamma2, and Bruton's tyrosine kinase. Thus, p110delta plays a critical role in B cell homeostasis and function.
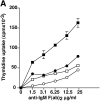
Figures









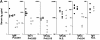
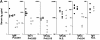
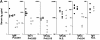
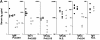











References
-
- Meffre, E., R. Casellas, and M.C. Nussenzweig. 2000. Antibody regulation of B cell development. Nat. Immunol. 1:379–385. - PubMed
-
- Craxton, A., K.L. Otipoby, A. Jiang, and E.A. Clark. 1999. Signal transduction pathways that regulate the fate of B lymphocytes. Adv. Immunol. 73:79–152. - PubMed
-
- Gold, M.R., V.W. Chan, C.W. Turck, and A.L. DeFranco. 1992. Membrane Ig cross-linking regulates phosphatidylinositol 3-kinase in B lymphocytes. J. Immunol. 148:2012–2022. - PubMed
-
- Yamanashi, Y., Y. Fukui, B. Wongsasant, Y. Kinoshita, Y. Ichimori, K. Toyoshima, and T. Yamamoto. 1992. Activation of Src-like protein-tyrosine kinase Lyn and its association with phosphatidylinositol 3-kinase upon B-cell antigen receptor-mediated signaling. Proc. Natl. Acad. Sci. USA. 89:1118–1122. - PMC - PubMed
-
- Tuveson, D.A., R.H. Carter, S.P. Soltoff, and D.T. Fearon. 1993. CD19 of B cells as a surrogate kinase insert region to bind phosphatidylinositol 3-kinase. Science. 260:986–989. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

