Fast synaptic inhibition promotes synchronized gamma oscillations in hippocampal interneuron networks
- PMID: 12235359
- PMCID: PMC130614
- DOI: 10.1073/pnas.192233099
Fast synaptic inhibition promotes synchronized gamma oscillations in hippocampal interneuron networks
Abstract
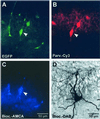
Networks of GABAergic interneurons are of critical importance for the generation of gamma frequency oscillations in the brain. To examine the underlying synaptic mechanisms, we made paired recordings from "basket cells" (BCs) in different subfields of hippocampal slices, using transgenic mice that express enhanced green fluorescent protein (EGFP) under the control of the parvalbumin promoter. Unitary inhibitory postsynaptic currents (IPSCs) showed large amplitude and fast time course with mean amplitude-weighted decay time constants of 2.5, 1.2, and 1.8 ms in the dentate gyrus, and the cornu ammonis area 3 (CA3) and 1 (CA1), respectively (33-34 degrees C). The decay of unitary IPSCs at BC-BC synapses was significantly faster than that at BC-principal cell synapses, indicating target cell-specific differences in IPSC kinetics. In addition, electrical coupling was found in a subset of BC-BC pairs. To examine whether an interneuron network with fast inhibitory synapses can act as a gamma frequency oscillator, we developed an interneuron network model based on experimentally determined properties. In comparison to previous interneuron network models, our model was able to generate oscillatory activity with higher coherence over a broad range of frequencies (20-110 Hz). In this model, high coherence and flexibility in frequency control emerge from the combination of synaptic properties, network structure, and electrical coupling.
Figures
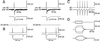


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

