The membrane trafficking protein calpactin forms a complex with bluetongue virus protein NS3 and mediates virus release
- PMID: 12235365
- PMCID: PMC130602
- DOI: 10.1073/pnas.192432299
The membrane trafficking protein calpactin forms a complex with bluetongue virus protein NS3 and mediates virus release
Abstract
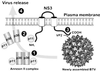
Bluetongue virus, an arbovirus of the Orbivirus genus, infects and replicates in both insect and mammalian cells. However, the cytopathic effect (cpe) on each host is very different. Mammalian cells show substantial cpe, most likely a result of the mechanism of virus release, whereas insect cells show little cpe and appear to release virus without cell lysis. Expression analysis of each infected cell type shows one protein, the nonstructural (NS) protein NS3, to be differentially expressed in the different cell types, suggesting it may act in the virus egress pathway. The molecular basis of such an interaction, however, has never been clear. Here, by using yeast two-hybrid analysis, we show that NS3 interacts with a cellular protein p11 (calpactin light chain), part of the annexin II complex that is involved in exocytosis. We map the NS3 region of interaction with p11 to a 13-residue peptide found at the N terminus of the protein and show it effectively competes with p36 (annexin II heavy chain) for p11 ligand binding. Further, we show that the C-terminal domain of NS3 interacts with VP2, the outermost protein of the fully assembled virus particle, suggesting that NS3 forms a bridging molecule that draws assembled virus into contact with the cellular export machinery. Our data describe the first host protein involvement in orbivirus egress and provide new insights into understanding arbovirus interactions with their hosts.
Figures






References
-
- Monath T P, Guirakhoo F. In: Fields Virology. 3rd Ed. Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Strauss S E, editors. Philadelphia: Lippincott–Raven; 1996. pp. 1735–1766.
-
- Jennings M, Boorman J. Arch Virol. 1979;59:121–126. - PubMed
-
- Homan E J, Yunker C E. Vet Microbiol. 1988;16:15–24. - PubMed
-
- Martin L A, Meyer A J, O'Hara R S, Fu H, Mellor P S, Knowles N J, Mertens P P. Arch Virol Suppl. 1998;14:281–293. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

