Secondary structure polymorphism in Oxytricha nova telomeric DNA
- PMID: 12235382
- PMCID: PMC137102
- DOI: 10.1093/nar/gkf517
Secondary structure polymorphism in Oxytricha nova telomeric DNA
Abstract
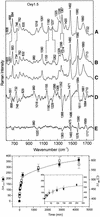
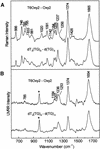
Tandem repeats of the telomeric DNA sequence d(T4G4) of Oxytricha nova are capable of forming unusually stable secondary structures incorporating Hoogsteen hydrogen bonding interactions. The biological significance of such DNA structures is supported by evidence of specific recognition of telomere end-binding proteins in the crystal state. To further characterize structural polymorphism of Oxytricha telomeric DNAs, we have obtained and interpreted Raman, ultraviolet resonance Raman (UVRR) and circular dichroism (CD) spectra of the tandem repeats d(G4T4G4) (Oxy1.5), d(T4G4)2 (Oxy2) and dT6(T4G4)2 (T6Oxy2) and related non-telomeric isomers in aqueous salt solutions. Raman markers of Oxy1.5 identify both C2'-endo/anti and C2'-endo/syn conformations of the deoxyguanosine residues and Hoogsteen hydrogen bonded guanine quartets, consistent with the quadruplex fold determined previously by solution NMR spectroscopy. Raman, UVRR and CD signatures and Raman dynamic measurements, to monitor imino NH-->ND exchanges, show that the Oxy1.5 antiparallel quadruplex fold is distinct from the hairpin structures of Oxy2 and T6Oxy2, single-stranded structures of d(TG)8 and dT6(TG)8 and previously reported quadruplex structures of d(T4G4)4 (Oxy4) and dG12. Spectral markers of the telomeric and telomere-related DNA structures are tabulated and novel Raman and UVRR indicators of thymidine and deoxyguanosine conformations are identified. The results will be useful for probing structures of Oxytricha telomeric repeats in complexes with telomere end-binding proteins.
Figures





Similar articles
-
A hairpin conformation for the 3' overhang of Oxytricha nova telomeric DNA.J Mol Biol. 1998 Aug 14;281(2):261-70. doi: 10.1006/jmbi.1998.1938. J Mol Biol. 1998. PMID: 9698547
-
Molecular mechanism of DNA recognition by the alpha subunit of the Oxytricha telomere binding protein.Biochemistry. 1999 Jan 12;38(2):582-8. doi: 10.1021/bi9819024. Biochemistry. 1999. PMID: 9888797
-
Structural basis of DNA recognition and mechanism of quadruplex formation by the beta subunit of the Oxytricha telomere binding protein.Biochemistry. 1998 Feb 3;37(5):1327-35. doi: 10.1021/bi972400d. Biochemistry. 1998. PMID: 9477960
-
G-quadruplexes as therapeutic targets.Biopolymers. 2000-2001;56(3):195-208. doi: 10.1002/1097-0282(2000)56:3<195::AID-BIP10009>3.0.CO;2-5. Biopolymers. 2000. PMID: 11745111 Review.
-
Structural identity of telomeric complexes.FEBS Lett. 2010 Sep 10;584(17):3785-99. doi: 10.1016/j.febslet.2010.08.004. Epub 2010 Aug 7. FEBS Lett. 2010. PMID: 20696167 Review.
Cited by
-
Vertebrate telomere repeat DNAs favor external loop propeller quadruplex structures in the presence of high concentrations of potassium.Nucleic Acids Res. 2005 Apr 7;33(6):2022-31. doi: 10.1093/nar/gki345. Print 2005. Nucleic Acids Res. 2005. PMID: 15817566 Free PMC article.
-
One identity or more for telomeres?Front Oncol. 2013 Mar 15;3:48. doi: 10.3389/fonc.2013.00048. eCollection 2013. Front Oncol. 2013. PMID: 23509004 Free PMC article.
-
Solvent Vibrations as a Proxy of the Telomere G-Quadruplex Rearrangements across Thermal Unfolding.Int J Mol Sci. 2022 May 4;23(9):5123. doi: 10.3390/ijms23095123. Int J Mol Sci. 2022. PMID: 35563512 Free PMC article.
-
Conformational changes in quadruplex oligonucleotide structures probed by Raman spectroscopy.Biomed Opt Express. 2010 Dec 23;2(2):207-17. doi: 10.1364/BOE.2.000207. Biomed Opt Express. 2010. PMID: 21339867 Free PMC article.
-
Optimization of Gonyautoxin1/4-Binding G-Quadruplex Aptamers by Label-Free Surface-Enhanced Raman Spectroscopy.Toxins (Basel). 2022 Sep 6;14(9):622. doi: 10.3390/toxins14090622. Toxins (Basel). 2022. PMID: 36136560 Free PMC article.
References
-
- McEachern M.J., Krauskopf,A. and Blackburn,E.H. (2000) Telomeres and their control. Annu. Rev. Genet., 34, 331–358. - PubMed
-
- Zakian V.A. (1995) Telomeres: beginning to understand the end. Science, 270, 1601–1607. - PubMed
-
- Zakian V.A. (1996) Structure, function and replication of Saccharomyces cerevisiae telomeres. Annu. Rev. Genet., 30, 141–172. - PubMed
-
- Baumann P. and Cech,T.R. (2001) Pot1, the putative telomere end-binding protein in fission yeast and humans. Science, 292, 1171–1175. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

