Antagonism of long-acting beta2-adrenoceptor agonism
- PMID: 12236842
- PMCID: PMC1874422
- DOI: 10.1046/j.1365-2125.2002.01651.x
Antagonism of long-acting beta2-adrenoceptor agonism
Abstract
The established place of regular long-acting beta2-adrenoceptor agonists at step 3 in asthma management guidelines has evolved as a consequence of evidence showing additive effects of salmeterol and formoterol on exacerbation rates, resulting in a putative inhaled corticosteroid sparing effect. There is however, evidence to show that although long-acting beta2-adrenoceptor agonists facilitate using a lower dose of inhaled corticosteroid, this may occur at the expense of suboptimal anti-inflammatory control. This is likely to be the case especially with fixed dose combination inhalers where it is not possible to properly titrate anti-inflammatory therapy with inhaled corticosteroids without also inadvertently overtreating with unnecessarily high doses of long-acting beta2-adrenoceptor agonists. Most patients with mild to moderate persistent asthma can be adequately controlled on monotherapy with inhaled corticosteroid in low or medium dosage, which is considerably cheaper than concomitant use of a long-acting beta2-adrenoceptor agonist. Subsensitivity to long-acting beta2-adrenoceptor agonists is a predictable pharmacological phenomenon which occurs despite concomitant inhaled corticosteroid therapy and occurs more readily for bronchoprotective than bronchodilator effects. Subsensitivity of salbutamol protection against bronchoconstrictor stimuli occurs in patients receiving concomitant long-acting beta2-adrenoceptor agonists, which may be due to beta2-adrenoceptor down-regulation or prolonged receptor occupancy. Prospective large scale long-term studies are required to further define the clinical relevance of beta2-adrenoceptor polymorphisms, to look at clinical control outcomes as well as propensity for subsensitivity. It would therefore make more sense to first of all optimize the dose of anti-inflammatory therapy with inhaled corticosteroid and to then consider adding a long-acting beta2-adrenoceptor agonist for patients who are poorly controlled.
Figures
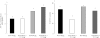
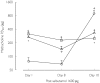
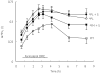
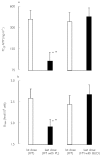
References
-
- The British Guidelines on asthma management. 1995 review and position statement. Thorax. 1997;52(Suppl 1):S1–S21.
-
- Lipworth BJ. Systemic adverse effects of inhaled corticosteroid therapy: a systematic review and meta-analysis. Arch Intern Med. 1999;159:941–955. - PubMed
-
- Sears MR, Taylor DR, Print CG, et al. Regular inhaled beta-agonist treatment in bronchial asthma. Lancet. 1990;336:1391–1396. - PubMed
-
- Spitzer WO, Suissa S, Ernst P, et al. The use of beta-agonists and the risk of death and near death from asthma. N Engl J Med. 1992;326:501–506. - PubMed

