Gradients of substrate-bound laminin orient axonal specification of neurons
- PMID: 12237407
- PMCID: PMC130496
- DOI: 10.1073/pnas.192457199
Gradients of substrate-bound laminin orient axonal specification of neurons
Abstract
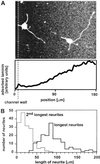
Little is known about the influence of substrate-bound gradients on neuronal development, since it has been difficult to fabricate gradients over the distances typically required for biological studies (a few hundred micrometers). This article demonstrates a generally applicable technique for the fabrication of substrate-bound gradients of proteins with complex shapes, using laminar flows in microchannels. Gradients that range from pure laminin to pure BSA were formed in solution by using a network of microchannels, and these proteins were allowed to adsorb onto a homogeneous layer of poly-l-lysine. Rat hippocampal neurons were cultivated on these substrate-bound gradients. Analysis of optical images of these neurons showed that axon specification is oriented in the direction of increasing surface density of laminin. Linear gradients in laminin adsorbed from a gradient in solution having a slope of nabla [laminin] > about 0.06 microg (ml.microm)(-1) (defined by dividing the change of concentration of laminin in solution over the distance of the gradient) orient axon specification, whereas those with nabla [laminin] < about 0.06 microg (ml.microm)(-1) have no effect.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

