Quantitative imaging of cis-regulatory reporters in living embryos
- PMID: 12237411
- PMCID: PMC130556
- DOI: 10.1073/pnas.202483199
Quantitative imaging of cis-regulatory reporters in living embryos
Abstract
A confocal laser scanning microscopy method has been developed for the quantitation of green fluorescent protein (GFP) as a reporter of gene activity in living three-dimensional structures such as sea urchin and starfish embryos. This method is between 2 and 50 times more accurate than conventional confocal microscopy procedures depending on the localization of GFP within an embryo. By using coinjected Texas red dextran as an internal fluorescent standard, the observed GFP intensity is corrected for variations in laser excitation and fluorescence collection efficiency. To relate the recorded image intensity to the number of GFP molecules, the embryos were lysed gently, and a fluorometric analysis of their contents was performed. Confocal laser scanning microscopy data collection from a single sea urchin blastula required less than 2 min, thereby allowing gene expression in dozens of embryos to be monitored in parallel with high spatial and temporal resolution.
Figures


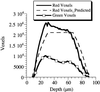
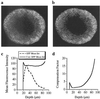
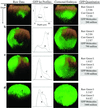
References
-
- Ferrer-Martinez A, Gomez-Foix A M. BioTechniques. 2002;32:62–66. - PubMed
-
- Davidson E H, Rast J P, Oliveri P, Ransick A, Calestani C, Yuh C-H, Minokawa T, Amore G, Hinman V, Arenas-Mena C, et al. Science. 2002;295:1669–1678. - PubMed
-
- Yuh C-H, Bolouri H, Davidson E H. Science. 1998;279:1896–1902. - PubMed
-
- Pawley J B, editor. Handbook of Biological Confocal Microscopy. New York: Plenum; 1995.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

