Molecular dynamics simulations of alanine rich beta-sheet oligomers: Insight into amyloid formation
- PMID: 12237456
- PMCID: PMC2373704
- DOI: 10.1110/ps.4270102
Molecular dynamics simulations of alanine rich beta-sheet oligomers: Insight into amyloid formation
Abstract
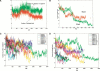
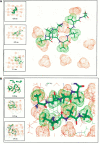
The aggregation observed in protein conformational diseases is the outcome of significant new beta-sheet structure not present in the native state. Peptide model systems have been useful in studies of fibril aggregate formation. Experimentally, it was found that a short peptide AGAAAAGA is one of the most highly amyloidogenic peptides. This peptide corresponds to the Syrian hamster prion protein (ShPrP) residues 113-120. The peptide was observed to be conserved in all species for which the PrP sequence has been determined. We have simulated the stabilities of oligomeric AGAAAAGA and AAAAAAAA (A8) by molecular dynamic simulations. Oligomers of both AGAAAAGA and AAAAAAAA were found to be stable when the size is 6 to 8 (hexamer to octamer). Subsequent simulation of an additional alpha-helical AAAAAAAA placed on the A8-octamer surface has revealed molecular events related to conformational change and oligomer growth. Our study addresses both the minimal oligomeric size of an aggregate seed and the mechanism of seed growth. Our simulations of the prion-derived 8-residue amyloidogenic peptide and its variant have indicated that an octamer is stable enough to be a seed and that the driving force for stabilization is the hydrophobic effect.
Figures








References
-
- Azriel, R. and Gazit, E. 2001. Analysis of the minimal amyloid-forming fragment of the islet amyloid polypeptide. J. Biol. Chem. 276 34156–34161. - PubMed
-
- Balbach, J.J., Ishii, Y., Antzutkin, O.N., Leapman, R.D., Rizzo, N.W., Dyda, F., Reed, J., and Tycko, R. 2000. Amyloid fibril formation by Aβ16–22, a seven-residue fragment of the Alzheimer's β-amyloid peptide, and structural characterization by solid state NMR. Biochemistry 39 13748–13759. - PubMed
-
- Benziger, T.L.S., Gregory, D.M. Burkoth, T.S., Miller-Auer, H., Lynn, D.G., Botto, R.E., and Meredith, S.C. 2000. Two-dimensional structure of β-amyloid(10–35) fibrils. Biochemistry 39 3491–3499. - PubMed
-
- Blondelle, S.B., Forood, B., Houghten, R.A., and Perez-Paya, E. 1996. Polyalanine-based peptides as models for self-associated β-pleated-sheet complexes. Biochemistry 36 8393–8400. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

