Topological investigation of amyloid fibrils obtained from beta2-microglobulin
- PMID: 12237458
- PMCID: PMC2373708
- DOI: 10.1110/ps.0206902
Topological investigation of amyloid fibrils obtained from beta2-microglobulin
Abstract
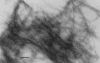
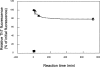
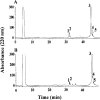
Amyloid fibrils of patients treated with regular hemodialysis essentially consists of beta2-microglobulin (beta2-m) and its truncated species DeltaN6beta2-m lacking six residues at the amino terminus. The truncated fragment has a more flexible three-dimensional structure and constitutes an excellent candidate for the analysis of a protein in the amyloidogenic conformation. The surface topology of synthetic fibrils obtained from intact beta2-m and truncated DeltaN6beta2-m was investigated by the limited proteolysis/mass spectrometry approach that appeared particularly suited to gain insights into the structure of beta2-m within the fibrillar polymer. The distribution of prefential proteolytic sites observed in both fibrils revealed that the central region of the protein, which had been easily cleaved in the full-length globular beta2-m, was fully protected in the fibrillar form. In addition, the amino- and carboxy-terminal regions of beta2-m became exposed to the solvent in the fibrils, whereas they were masked completely in the native protein. These data indicate that beta2-m molecules in the fibrils consist of an unaccessible core comprising residues 20-87 with the strands I and VIII being not constrained in the fibrillar polymer and exposed to the proteases. Moreover, proteolytic cleavages observed in vitro at Lys 6 and Lys 19 reproduce specific cleavages that have to occur in vivo to generate the truncated forms of beta2-m occurring in natural fibrils. On the basis of these data, a possible mechanism for fibril formation from native beta2-m is discussed and an explanation for the occurrence of truncated protein species in natural fibrils is given.
Figures


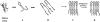
Similar articles
-
Limited proteolysis in the investigation of beta2-microglobulin amyloidogenic and fibrillar states.Biochim Biophys Acta. 2005 Nov 10;1753(1):44-50. doi: 10.1016/j.bbapap.2005.09.004. Epub 2005 Sep 23. Biochim Biophys Acta. 2005. PMID: 16213198 Review.
-
Core and heterogeneity of beta2-microglobulin amyloid fibrils as revealed by H/D exchange.J Mol Biol. 2004 Apr 30;338(3):559-71. doi: 10.1016/j.jmb.2004.02.067. J Mol Biol. 2004. PMID: 15081813
-
Removal of the N-terminal hexapeptide from human beta2-microglobulin facilitates protein aggregation and fibril formation.Protein Sci. 2000 May;9(5):831-45. doi: 10.1110/ps.9.5.831. Protein Sci. 2000. PMID: 10850793 Free PMC article.
-
Heparin strongly enhances the formation of beta2-microglobulin amyloid fibrils in the presence of type I collagen.J Biol Chem. 2008 Feb 22;283(8):4912-20. doi: 10.1074/jbc.M702712200. Epub 2007 Dec 3. J Biol Chem. 2008. PMID: 18056266
-
Molecular interactions in the formation and deposition of beta2-microglobulin-related amyloid fibrils.Amyloid. 2005 Mar;12(1):15-25. doi: 10.1080/13506120500032352. Amyloid. 2005. PMID: 16076607 Review.
Cited by
-
The Early Phase of β2-Microglobulin Aggregation: Perspectives From Molecular Simulations.Front Mol Biosci. 2020 Sep 29;7:578433. doi: 10.3389/fmolb.2020.578433. eCollection 2020. Front Mol Biosci. 2020. PMID: 33134317 Free PMC article. Review.
-
Investigating the structural properties of amyloid-like fibrils formed in vitro from beta2-microglobulin using limited proteolysis and electrospray ionisation mass spectrometry.Rapid Commun Mass Spectrom. 2006;20(11):1628-36. doi: 10.1002/rcm.2482. Rapid Commun Mass Spectrom. 2006. PMID: 16636995 Free PMC article.
-
Ribosomal protein L7a binds RNA through two distinct RNA-binding domains.Biochem J. 2005 Jan 1;385(Pt 1):289-99. doi: 10.1042/BJ20040371. Biochem J. 2005. PMID: 15361074 Free PMC article.
-
Structure and dynamics of oligomeric intermediates in β2-microglobulin self-assembly.Biophys J. 2011 Sep 7;101(5):1238-47. doi: 10.1016/j.bpj.2011.07.023. Biophys J. 2011. PMID: 21889462 Free PMC article.
-
Towards an understanding of the structural molecular mechanism of beta(2)-microglobulin amyloid formation in vitro.Biochim Biophys Acta. 2005 Nov 10;1753(1):51-63. doi: 10.1016/j.bbapap.2005.07.006. Epub 2005 Aug 15. Biochim Biophys Acta. 2005. PMID: 16099226 Free PMC article. Review.
References
-
- Atkinson, R.A., Joseph, C., Dal Piaz, F., Birolo, L., Stier, G., Pucci, P., and Pastore A. 2000. Binding of α-actinin to titin: Implications for Z-disk assembly.Biochemistry 39 5255–5264. - PubMed
-
- Bellotti, V., Stoppini, M., Mangione, P., Sunde, M., Robinson, C.V., Asti, L., Brancaccio, D., and Ferri, G. 1998. β2-microglobulin can be refolded into a native state from ex vivo amyloid fibrils.Eur. J. Biochem. 258 355–560. - PubMed
-
- Bianchi, E., Orru, S., Dal Piaz, F., Ingenito, R., Casbarra, A., Biasiol, G., Koch, U., Pucci, P., and Pessi, A. 1999. Conformational changes in human hepatitis C virus NS3 protease upon binding of product-based inhibitors.Biochemistry. 38 13844–13852. - PubMed
-
- Chiti, F., Mangione, P., Andreola, A., Giorgetti, S., Stefani, M., Dobson, C.M., Bellotti, V., and Taddei, N. 2001a. Detection of two partially structured species in the folding process of the amyloidogenic protein β 2-microglobulin.J. Mol. Biol.. 307 379–391. - PubMed
-
- Chiti, F., De Lorenzi, E., Grossi, S., Mangione, P., Giorgetti, S., Caccialanza, G., Dobson, C.M., Merlini, G., Ramponi, G., and Bellotti V. 2001b. A partially structured species of β 2-microglobulin is significantly populated under physiological conditions and involved in fibrillogenesis.J. Biol. Chem. 276 46714–46721. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

