Molecular modeling-based analysis of interactions in the RFC-dependent clamp-loading process
- PMID: 12237462
- PMCID: PMC2373712
- DOI: 10.1110/ps.0214302
Molecular modeling-based analysis of interactions in the RFC-dependent clamp-loading process
Abstract
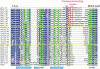
Replication and related processes in eukaryotic cells require replication factor C (RFC) to load a molecular clamp for DNA polymerase in an ATP-driven process, involving multiple molecular interactions. The detailed understanding of this mechanism is hindered by the lack of data regarding structure, mutual arrangement, and dynamics of the players involved. In this study, we analyzed interactions that take place during loading onto DNA of either the PCNA clamp or the Rad9-Rad1-Hus1 checkpoint complex, using computationally derived molecular models. Combining the modeled structures for each RFC subunit with known structural, biochemical, and genetic data, we propose detailed models of how two of the RFC subunits, RFC1 and RFC3, interact with the C-terminal regions of PCNA. RFC1 is predicted to bind PCNA similarly to the p21-PCNA interaction, while the RFC3-PCNA binding is proposed to be similar to the E. coli delta-beta interaction. Additional sequence and structure analysis, supported by experimental data, suggests that RFC5 might be the third clamp loader subunit to bind the equivalent PCNA region. We discuss functional implications stemming from the proposed model of the RFC1-PCNA interaction and compare putative clamp-interacting regions in RFC1 and its paralogs, Rad17 and Ctf18. Based on the individual intermolecular interactions, we propose RFC and PCNA arrangement that places three RFC subunits in association with each of the three C-terminal regions in PCNA. The two other RFC subunits are positioned at the two PCNA interfaces, with the third PCNA interface left unobstructed. In addition, we map interactions at the level of individual subunits between the alternative clamp loader/clamp system, Rad17-RFC(2-5)/Rad9-Rad1-Hus1. The proposed models of interaction between two clamp/clamp loader pairs provide both structural framework for interpretation of existing experimental data and a number of specific findings that can be subjected to direct experimental testing.
Figures
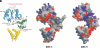


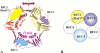
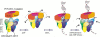
Similar articles
-
Structure-based predictions of Rad1, Rad9, Hus1 and Rad17 participation in sliding clamp and clamp-loading complexes.Nucleic Acids Res. 2000 Jul 1;28(13):2481-93. doi: 10.1093/nar/28.13.2481. Nucleic Acids Res. 2000. PMID: 10871397 Free PMC article.
-
The PCNA-RFC families of DNA clamps and clamp loaders.Prog Nucleic Acid Res Mol Biol. 2004;78:227-60. doi: 10.1016/S0079-6603(04)78006-X. Prog Nucleic Acid Res Mol Biol. 2004. PMID: 15210332 Review.
-
Biochemical characterization of DNA damage checkpoint complexes: clamp loader and clamp complexes with specificity for 5' recessed DNA.PLoS Biol. 2003 Nov;1(2):E33. doi: 10.1371/journal.pbio.0000033. Epub 2003 Nov 17. PLoS Biol. 2003. PMID: 14624239 Free PMC article.
-
Functions of Multiple Clamp and Clamp-Loader Complexes in Eukaryotic DNA Replication.Adv Exp Med Biol. 2017;1042:135-162. doi: 10.1007/978-981-10-6955-0_7. Adv Exp Med Biol. 2017. PMID: 29357057 Review.
-
Cryo-EM reveals a nearly complete PCNA loading process and unique features of the human alternative clamp loader CTF18-RFC.Proc Natl Acad Sci U S A. 2024 Apr 30;121(18):e2319727121. doi: 10.1073/pnas.2319727121. Epub 2024 Apr 26. Proc Natl Acad Sci U S A. 2024. PMID: 38669181 Free PMC article.
Cited by
-
Fission yeast strains with circular chromosomes require the 9-1-1 checkpoint complex for the viability in response to the anti-cancer drug 5-fluorodeoxyuridine.PLoS One. 2017 Nov 9;12(11):e0187775. doi: 10.1371/journal.pone.0187775. eCollection 2017. PLoS One. 2017. PMID: 29121084 Free PMC article.
-
A structure-function analysis of the yeast Elg1 protein reveals the importance of PCNA unloading in genome stability maintenance.Nucleic Acids Res. 2017 Apr 7;45(6):3189-3203. doi: 10.1093/nar/gkw1348. Nucleic Acids Res. 2017. PMID: 28108661 Free PMC article.
-
Stepwise loading of yeast clamp revealed by ensemble and single-molecule studies.Proc Natl Acad Sci U S A. 2010 Nov 16;107(46):19736-41. doi: 10.1073/pnas.1014139107. Epub 2010 Nov 1. Proc Natl Acad Sci U S A. 2010. PMID: 21041673 Free PMC article.
-
DNA replication factor C1 mediates genomic stability and transcriptional gene silencing in Arabidopsis.Plant Cell. 2010 Jul;22(7):2336-52. doi: 10.1105/tpc.110.076349. Epub 2010 Jul 16. Plant Cell. 2010. PMID: 20639449 Free PMC article.
-
Genome-wide analysis of the core DNA replication machinery in the higher plants Arabidopsis and rice.Plant Physiol. 2007 Aug;144(4):1697-714. doi: 10.1104/pp.107.101105. Epub 2007 Jun 7. Plant Physiol. 2007. PMID: 17556508 Free PMC article.
References
-
- Bower, M.J., Cohen, F.E., and Dunbrack Jr., R.L. 1997. Prediction of protein side-chain rotamers from a backbone-dependent rotamer library: A new homology modeling tool.J. Mol. Biol. 267 1268–1282. - PubMed
-
- Burtelow, M.A., Roos-Mattjus, P.M., Rauen, M., Babendure, J.R., and Karnitz, L.M. 2001. Reconstitution and molecular analysis of the hRad9-hHus1-hRad1 (9–1–1) DNA damage responsive checkpoint complex.J. Biol. Chem. 276 25903–25909. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

