Changing the net charge from negative to positive makes ribonuclease Sa cytotoxic
- PMID: 12237473
- PMCID: PMC2373699
- DOI: 10.1110/ps.0216702
Changing the net charge from negative to positive makes ribonuclease Sa cytotoxic
Abstract
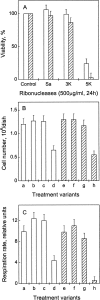
Ribonuclease Sa (pI = 3.5) from Streptomyces aureofaciens and its 3K (D1K, D17K, E41K) (pI = 6.4) and 5K (3K + D25K, E74K) (pI = 10.2) mutants were tested for cytotoxicity. The 5K mutant was cytotoxic to normal and v-ras-transformed NIH3T3 mouse fibroblasts, but RNase Sa and 3K were not. The structure, stability, and activity of the three proteins are comparable, but the net charge at pH 7 increases from -7 for RNase Sa to -1 for 3K and to +3 for 5K. These results suggest that a net positive charge is a key determinant of ribonuclease cytotoxicity. The cytotoxic 5K mutant preferentially attacks v-ras-NIH3T3 fibroblasts, suggesting that mammalian cells expressing the ras-oncogene are potential targets for ribonuclease-based drugs.
Figures
References
-
- Bishop, J.M. 1991. Molecular themes in oncogenesis. Cell 64 235–248. - PubMed
-
- Bretscher, L.E., Abel, R.L., and Raines, R.T. 2000. A ribonuclease A variant with low catalytic activity but high cytotoxicity. J. Biol. Chem. 275 9893–9896. - PubMed
-
- Futami, J., Maeda, T., Kitazoe, M., Nukui, E,. Tada, H., Seno, M., Kosaka, M., and Yamada, H. 2001. Preparation of potent cytotoxic ribonuclease by cationization: Enhanced cellular uptake and decreased interaction with ribonuclease inhibitor by chemical modification of carboxyl groups. Biochemistry 40 7518–7524. - PubMed
-
- Hebert, E.J., Grimsley, G.R., Hartley, R.W., Horn, G., Schell, D., Garcia, S., Both, V., Sevcik, J., and Pace, C.N. 1997. Purification of ribonucleases Sa, Sa2, and Sa3 after expression in Escherichia coli. Protein Expr. Purif. 11 162–168. - PubMed
-
- Ilinskaya, O.N. and Vamvakas, S. 1997. Nephrotoxic effects of bacterial ribonucleases in the isolated perfused rat kidney. Toxicology 120 55–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

