Rho GTPases in human breast tumours: expression and mutation analyses and correlation with clinical parameters
- PMID: 12237774
- PMCID: PMC2364248
- DOI: 10.1038/sj.bjc.6600510
Rho GTPases in human breast tumours: expression and mutation analyses and correlation with clinical parameters
Abstract
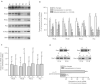
In the present study, we addressed the question of a putative relevance of Rho proteins in tumour progression by analysing their expression on protein and mRNA level in breast tumours. We show that the level of RhoA, RhoB, Rac1 and Cdc42 protein is largely enhanced in all tumour samples analysed (n=15) as compared to normal tissues originating from the same individual. The same is true for (32)P-ADP-ribosylation of Rho proteins which is catalysed by Clostridium botulinum exoenzyme C3. Also the amount of Rho-GDI and ERK2 as well as the level of overall (32)P-GTP binding activity was tumour-specific elevated, yet to a lower extent than Rho proteins. Although the amount of Rho proteins was enhanced in tumours, most of them did not show changes in rho mRNA expression as compared to the corresponding normal tissue. Thus, elevated gene expression seems not to be the underlying mechanism of tumour-specific overexpression of Rho proteins. Sequence analysis of RhoA, RhoB, RhoC and Rac1 failed to detect any mutations in both the GTP-binding site and effector binding region. By analysing >50 tumour samples, the amount of RhoA-like proteins (i.e. RhoA, B, C), but not of Rac1, was found to significantly increase with histological grade and proliferation index. Rho protein expression was neither related to p53 nor to HER-2/neu oncogene status. Expression of rho mRNAs did not show a significant increase with histological grade. Overall the data show that (1) Rho proteins are overexpressed in breast tumours (2) overexpression is not regulated on the mRNA level (3) the expression level of RhoA-like proteins correlates with malignancy and (4) Rho proteins are not altered by mutation in breast tumours.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

