Adaptation of alphaviruses to heparan sulfate: interaction of Sindbis and Semliki forest viruses with liposomes containing lipid-conjugated heparin
- PMID: 12239287
- PMCID: PMC136541
- DOI: 10.1128/jvi.76.20.10128-10137.2002
Adaptation of alphaviruses to heparan sulfate: interaction of Sindbis and Semliki forest viruses with liposomes containing lipid-conjugated heparin
Abstract
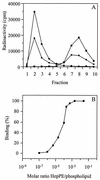
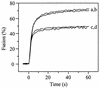
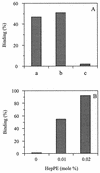
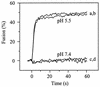
Passage of Sindbis virus (SIN) in BHK-21 cells has been shown to select for virus mutants with high affinity for the glycosaminoglycan heparan sulfate (HS). Three loci in the viral spike protein E2 (E2:1, E2:70, and E2:114) have been identified that mutate during adaptation and independently confer on the virus the ability to bind to cell surface HS (W. B. Klimstra, K. D. Ryman, and R. E. Johnston, J. Virol. 72:7357-7366, 1998). In this study, we used HS-adapted SIN mutants to evaluate a new model system involving target liposomes containing lipid-conjugated heparin (HepPE) as an HS receptor analog for the virus. HS-adapted SIN, but not nonadapted wild-type SIN TR339, interacted efficiently with HepPE-containing liposomes at neutral pH. Binding was competitively inhibited by soluble heparin. Despite the efficient binding of HS-adapted SIN to HepPE-containing liposomes at neutral pH, there was no fusion under these conditions. Fusion did occur, however, at low pH, consistent with cellular entry of the virus via acidic endosomes. At low pH, wild-type or HS-adapted SIN underwent fusion with liposomes with or without HepPE with similar kinetics, suggesting that interaction with the HS receptor analog at neutral pH has little influence on subsequent fusion of SIN at low pH. Finally, Semliki Forest virus (SFV), passaged frequently on BHK-21 cells, also interacted efficiently with HepPE-containing liposomes, indicating that SFV, like other alphaviruses, readily adapts to cell surface HS. In conclusion, the liposomal model system presented in this paper may serve as a novel tool for the study of receptor interactions and membrane fusion properties of HS-interacting enveloped viruses.
Figures





References
-
- Bernard, K. A., W. B. Klimstra, and R. E. Johnston. 2000. Mutations in the E2 glycoprotein of Venezuelan equine encephalitis virus confer heparan sulfate interaction, low morbidity, and rapid clearance from blood of mice. Virology 276:93-103. - PubMed
-
- Bernfield, M., M. Götte, P. W. Park, O. Reizes, M. L. Fitzgerald, J. Lincecum, and M. Zako. 1999. Functions of cell surface heparan sulfate proteoglycans. Annu. Rev. Biochem. 68:729-777. - PubMed
-
- Böttcher, C. J. F., C. M. van Gent, and C. Fries. 1961. A rapid and sensitive sub-micro phosphorus determination. Anal. Chim. Acta 24:203-204.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

