A P22 scaffold protein mutation increases the robustness of head assembly in the presence of excess portal protein
- PMID: 12239300
- PMCID: PMC136566
- DOI: 10.1128/jvi.76.20.10245-10255.2002
A P22 scaffold protein mutation increases the robustness of head assembly in the presence of excess portal protein
Abstract
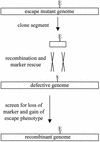
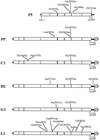
Bacteriophage with linear, double-stranded DNA genomes package DNA into preassembled protein shells called procapsids. Located at one vertex in the procapsid is a portal complex composed of a ring of 12 subunits of portal protein. The portal complex serves as a docking site for the DNA packaging enzymes, a conduit for the passage of DNA, and a binding site for the phage tail. An excess of the P22 portal protein alters the assembly pathway of the procapsid, giving rise to defective procapsid-like particles and aberrant heads. In the present study, we report the isolation of escape mutant phage that are able to replicate more efficiently than wild-type phage in the presence of excess portal protein. The escape mutations all mapped to the same phage genome segment spanning the portal, scaffold, coat, and open reading frame 69 genes. The mutations present in five of the escape mutants were determined by DNA sequencing. Interestingly, each mutant contained the same mutation in the scaffold gene, which changes the glycine at position 287 to glutamate. This mutation alone conferred an escape phenotype, and the heads assembled by phage harboring only this mutation had reduced levels of portal protein and exhibited increased head assembly fidelity in the presence of excess portal protein. Because this mutation resides in a region of scaffold protein necessary for coat protein binding, these findings suggest that the P22 scaffold protein may define the portal vertices in an indirect manner, possibly by regulating the fidelity of coat protein polymerization.
Figures




References
-
- Bazinet, C., and J. King. 1985. The DNA translocating vertex of dsDNA bacteriophage. Annu. Rev. Microbiol. 39:109-129. - PubMed
-
- Bazinet, C., and J. King. 1988. Initiation of P22 procapsid assembly in vivo. J. Mol. Biol. 202:77-86. - PubMed
-
- Bazinet, C., R. Villafane, and J. King. 1990. Novel second-site suppression of a cold-sensitive defect in phage P22 procapsid assembly. J. Mol. Biol. 216:701-716. - PubMed
-
- Botstein, D., and M. Levine. 1968. Intermediates in the synthesis of phage P22 DNA. Cold Spring Harbor Symp. Quant. Biol. 33:659-667. - PubMed
-
- Botstein, D., C. H. Waddell, and J. King. 1973. Mechanism of head assembly and DNA encapsulation in Salmonella phage p22. I. Genes, proteins, structures and DNA maturation. J. Mol. Biol. 80:669-695. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

