Signal Transduction and Transcription Factor Modification during Reactivation of Epstein-Barr Virus from Latency
- PMID: 12239305
- PMCID: PMC136559
- DOI: 10.1128/jvi.76.20.10290-10298.2002
Signal Transduction and Transcription Factor Modification during Reactivation of Epstein-Barr Virus from Latency
Abstract
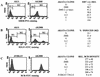
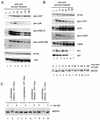
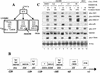
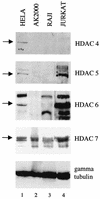
Reactivation of Epstein-Barr virus (EBV) from latency involves activation of the Zp promoter of the EBV BZLF1 gene. This occurs rapidly and efficiently in response to cross-linking the B-cell receptor on Akata Burkitt's lymphoma cells. After optimizing conditions for induction, signal transduction responses to B-cell receptor cross-linking were observed within 10 min, well before any autoactivation effects of BZLF1 protein. The primary events in reactivation were shown to involve dephosphorylation of the myocyte enhancer factor 2D (MEF-2D) transcription factor via the cyclosporin A-sensitive, calcium-mediated signaling pathway. This and other signal transduction events were correlated with the quantitative promoter analysis reported in the accompanying paper (U. K. Binné, W. Amon, and P. J. Farrell, this issue). Dephosphorylation of MEF-2D is known to be associated with histone acetylase recruitment, correlating with the histone acetylation at Zp during reactivation that we reported previously (Jenkins et al., J. Virol. 74:710-720, 2000). Histone deacetylation in response to phosphorylated MEF-2D can be mediated by class I or class II histone deacetylases (HDACs); HDAC 7 was the most readily detected class II HDAC in Akata and Raji cells, suggesting that it may be involved in Zp repression during latency.
Figures


References
-
- Babcock, G. J., D. Hochberg, and A. D. Thorley-Lawson. 2000. The expression pattern of Epstein-Barr virus latent genes in vivo is dependent upon the differentiation stage of the infected B cell. Immunity 13:497-506. - PubMed
-
- Bauer, G., P. Hofler, and M. Simon. 1982. Epstein-Barr virus induction by a serum factor. Characterization of the purified factor and the mechanism of its activation. J. Biol. Chem. 257:11411-11415. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

