Human T-cell lymphotropic virus type 1 p12(I) expression increases cytoplasmic calcium to enhance the activation of nuclear factor of activated T cells
- PMID: 12239314
- PMCID: PMC136546
- DOI: 10.1128/jvi.76.20.10374-10382.2002
Human T-cell lymphotropic virus type 1 p12(I) expression increases cytoplasmic calcium to enhance the activation of nuclear factor of activated T cells
Abstract
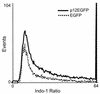
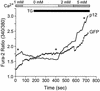
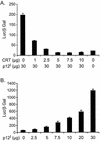
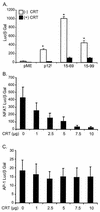
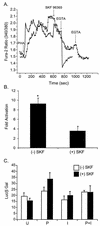
Human T-cell lymphotropic virus type 1 (HTLV-1) establishes persistent infection and is associated with lymphoproliferative or neurodegenerative diseases. As a complex retrovirus, HTLV-1 contains typical structural and enzymatic genes, as well as regulatory and accessory genes encoded in the pX region. The early events necessary for HTLV-1 to establish infection in lymphocytes, its primary target cells, remain unresolved. Recent studies have demonstrated the importance of regulatory and accessory gene products in determining this virus-host interaction. Among these, pX open reading frame I, which encodes two proteins, p12(I) and p27(I), is required for establishing persistent infection in vivo and for infection in quiescent primary lymphocytes. In addition, p12(I) localizes in the endoplasmic reticulum (ER) and cis-Golgi apparatus and associates with a calcium binding protein, calreticulin. We recently reported that p12(I) expression induces the calcium-responsive T-cell transcription factor, nuclear factor of activated T cells (NFAT), in the presence of phorbol ester activation. Based on these studies, we hypothesize that p12(I) may modulate calcium release from the ER. Here, we report that p12(I) expression increases basal cytoplasmic calcium and concurrently diminishes calcium available for release from the ER stores. Overexpression of calreticulin, a calcium buffer protein, blocked p12(I)-mediated NFAT activation independently of its ability to bind p12(I). Chemical inhibition studies using inhibitors of inositol 1,4,5-triphosphate receptor and calcium release-activated calcium channels suggest that inositol 1,4,5-triphosphate receptor in the ER membrane and calcium release-activated calcium channels in the plasma membrane contribute to p12(I)-mediated NFAT activation. Collectively, our results are the first to demonstrate the role of p12(I) in elevating cytoplasmic calcium, an antecedent to T-cell activation, and further support the important role of this accessory protein in the early events of HTLV-1 infection.
Figures



Similar articles
-
Activation of nuclear factor of activated T cells by human T-lymphotropic virus type 1 accessory protein p12(I).J Virol. 2002 Apr;76(7):3493-501. doi: 10.1128/jvi.76.7.3493-3501.2002. J Virol. 2002. PMID: 11884573 Free PMC article.
-
Human T-cell lymphotropic virus type 1 p12I enhances interleukin-2 production during T-cell activation.J Virol. 2003 Oct;77(20):11027-39. doi: 10.1128/jvi.77.20.11027-11039.2003. J Virol. 2003. PMID: 14512551 Free PMC article.
-
Endoplasmic reticulum and cis-Golgi localization of human T-lymphotropic virus type 1 p12(I): association with calreticulin and calnexin.J Virol. 2001 Aug;75(16):7672-82. doi: 10.1128/JVI.75.16.7672-7682.2001. J Virol. 2001. PMID: 11462039 Free PMC article.
-
Critical role of human T-lymphotropic virus type 1 accessory proteins in viral replication and pathogenesis.Microbiol Mol Biol Rev. 2002 Sep;66(3):396-406, table of contents. doi: 10.1128/MMBR.66.3.396-406.2002. Microbiol Mol Biol Rev. 2002. PMID: 12208996 Free PMC article. Review.
-
Role of accessory proteins of HTLV-1 in viral replication, T cell activation, and cellular gene expression.Front Biosci. 2004 Sep 1;9:2556-76. doi: 10.2741/1417. Front Biosci. 2004. PMID: 15358581 Free PMC article. Review.
Cited by
-
The SCHOOL of nature: IV. Learning from viruses.Self Nonself. 2010 Oct;1(4):282-298. doi: 10.4161/self.1.4.13279. Self Nonself. 2010. PMID: 21487503 Free PMC article.
-
HTLV-1 ORF-I Encoded Proteins and the Regulation of Host Immune Response: Viral Induced Dysregulation of Intracellular Signaling.J Immunol Res. 2015;2015:498054. doi: 10.1155/2015/498054. Epub 2015 Oct 18. J Immunol Res. 2015. PMID: 26557721 Free PMC article. Review.
-
Animal models for human T-lymphotropic virus type 1 (HTLV-1) infection and transformation.Oncogene. 2005 Sep 5;24(39):6005-15. doi: 10.1038/sj.onc.1208974. Oncogene. 2005. PMID: 16155607 Free PMC article. Review.
-
NK cells and monocytes modulate primary HTLV-1 infection.PLoS Pathog. 2022 Apr 4;18(4):e1010416. doi: 10.1371/journal.ppat.1010416. eCollection 2022 Apr. PLoS Pathog. 2022. PMID: 35377924 Free PMC article.
-
SCHOOL model and new targeting strategies.Adv Exp Med Biol. 2008;640:268-311. doi: 10.1007/978-0-387-09789-3_20. Adv Exp Med Biol. 2008. PMID: 19065798 Free PMC article. Review.
References
-
- Berneman, Z. N., R. B. Gartenhaus, M. S. Reitz, W. A. Blattner, A. Manns, B. Hanchard, O. Ikehara, R. C. Gallo, and M. E. Klotman. 1992. Expression of alternatively spliced human T-lymphotropic virus type 1 pX mRNA in infected cell lines and in primary uncultured cells from patients with adult T-cell leukemia/lymphoma and healthy carriers. Proc. Natl. Acad. Sci. USA 89:3005-3009. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

