Life cycle heterogeneity in animal models of human papillomavirus-associated disease
- PMID: 12239317
- PMCID: PMC136551
- DOI: 10.1128/jvi.76.20.10401-10416.2002
Life cycle heterogeneity in animal models of human papillomavirus-associated disease
Abstract
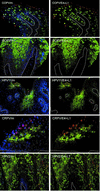
Animal papillomaviruses are widely used as models to study papillomavirus infection in humans despite differences in genome organization and tissue tropism. Here, we have investigated the extent to which animal models of papillomavirus infection resemble human disease by comparing the life cycles of 10 different papillomavirus types. Three phases in the life cycles of all viruses were apparent using antibodies that distinguish between early events, the onset of viral genome amplification, and the expression of capsid proteins. The initiation of these phases follows a highly ordered pattern that appears important for the production of virus particles. The viruses examined included canine oral papillomavirus, rabbit oral papillomavirus (ROPV), cottontail rabbit papillomavirus (CRPV), bovine papillomavirus type 1, and human papillomavirus types 1, 2, 11, and 16. Each papillomavirus type showed a distinctive gene expression pattern that could be explained in part by differences in tissue tropism, transmission route, and persistence. As the timing of life cycle events affects the accessibility of viral antigens to the immune system, the ideal model system should resemble human mucosal infection if vaccine design is to be effective. Of the model systems examined here, only ROPV had a tissue tropism and a life cycle organization that resembled those of the human mucosal types. ROPV appears most appropriate for studies of the life cycles of mucosal papillomavirus types and for the development of prophylactic vaccines. The persistence of abortive infections caused by CRPV offers advantages for the development of therapeutic vaccines.
Figures







References
-
- Ashmole, I., P. H. Gallimore, and S. Roberts. 1998. Identification of conserved hydrophobic C-terminal residues of the human papillomavirus type 1 E1E4 protein necessary for E4 oligomerisation in vivo. Virology 240:221-231. - PubMed
-
- Barrera-Oro, J. G., K. O. Smith, and J. L. Melnick. 1962. Quantitation of papova virus particles in human warts. J. Natl. Cancer Inst. 29:583-595. - PubMed
-
- Bell, J. A., J. P. Sundberg, S. J. Ghim, J. Newsome, A. B. Jenson, and R. Schlegel. 1994. A formalin-inactivated vaccine protects against mucosal papillomavirus infection: a canine model. Pathobiology 62:194-198. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

