Influenza virus can enter and infect cells in the absence of clathrin-mediated endocytosis
- PMID: 12239322
- PMCID: PMC136567
- DOI: 10.1128/jvi.76.20.10455-10464.2002
Influenza virus can enter and infect cells in the absence of clathrin-mediated endocytosis
Abstract
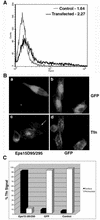
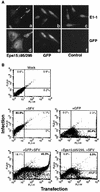
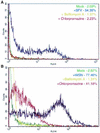
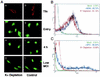
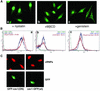
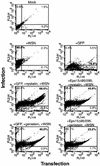
Influenza virus has been described to enter host cells via clathrin-mediated endocytosis. However, it has also been suggested that other endocytic routes may provide additional entry pathways. Here we show that influenza virus may enter and infect HeLa cells that are unable to take up ligands by clathrin-mediated endocytosis. By overexpressing a dominant-negative form of the Eps15 protein to inhibit clathrin-mediated endocytosis, we demonstrate that while transferrin uptake and Semliki Forest virus infection were prevented, influenza virus could enter and infect cells expressing Eps15Delta95/295. This finding is supported by the successful infection of cells with influenza virus in the presence of chemical treatments that block endocytosis, namely, chlorpromazine and potassium depletion. We show also that influenza virus may infect cells incapable of uptake by caveolae. Treatment with the inhibitors nystatin, methyl-beta-cyclodextrin, and genistein, as well as transfection of cells with dominant-negative caveolin-1, had no effect on influenza virus infection. By combining inhibitory methods to block both clathrin-mediated endocytosis and uptake by caveolae in the same cell, we demonstrate that influenza virus may infect cells by an additional non-clathrin-dependent, non-caveola-dependent endocytic pathway. We believe this to be the first conclusive analysis of virus entry via such a non-clathrin-dependent pathway, in addition to the traditional clathrin-dependent route.
Figures


References
-
- Bayer, N., D. Schober, M. Huttinger, D. Blaas, and R. Fuchs. 2001. Inhibition of clathrin-dependent endocytosis has multiple effects on human rhinovirus serotype 2 cell entry. J. Biol. Chem. 276:3952-3962. - PubMed
-
- Benmerah, A., M. Bayrou, N. Cerf-Bensussan, and A. Dautry-Varsat. 1999. Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J. Cell Sci. 112:1303-1311. - PubMed
-
- Benmerah, A., B. Begue, A. Dautry-Varsat, and N. Cerf-Bensussan. 1996. The ear of alpha-adaptin interacts with the COOH-terminal domain of the Eps 15 protein. J. Biol. Chem. 271:12111-12116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

