Protective antiviral immune responses to pseudorabies virus induced by DNA vaccination using dimethyldioctadecylammonium bromide as an adjuvant
- PMID: 12239334
- PMCID: PMC136542
- DOI: 10.1128/jvi.76.20.10540-10545.2002
Protective antiviral immune responses to pseudorabies virus induced by DNA vaccination using dimethyldioctadecylammonium bromide as an adjuvant
Abstract
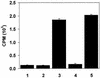
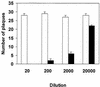
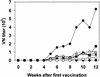
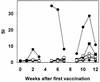
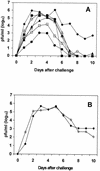
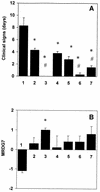
To enhance the efficacy of a DNA vaccine against pseudorabies virus (PRV), we evaluated the adjuvant properties of plasmids coding for gamma interferon or interleukin-12, of CpG immunostimulatory motifs, and of the conventional adjuvants dimethyldioctadecylammonium bromide in water (DDA) and sulfolipo-cyclodextrin in squalene in water. We demonstrate that a DNA vaccine combined with DDA, but not with the other adjuvants, induced significantly stronger immune responses than plasmid vaccination alone. Moreover, pigs vaccinated in the presence of DDA were protected against clinical disease and shed significantly less PRV after challenge infection. This is the first study to demonstrate that DDA, a conventional adjuvant, enhances DNA vaccine-induced antiviral immunity.
Figures
References
-
- Bouma, A., R. J. Zwart, M. G. de Bruin, M. C. de Jong, T. G. Kimman, and A. T. Bianchi. 1997. Immunohistological characterization of the local cellular response directed against pseudorabies virus in pigs. Vet. Microbiol. 58:145-154. - PubMed
-
- Chow, Y. H., B. L. Chiang, Y. L. Lee, W. K. Chi, W. C. Lin, Y. T. Chen, and M. H. Tao. 1998. Development of Th1 and Th2 populations and the nature of immune responses to hepatitis B virus DNA vaccines can be modulated by codelivery of various cytokine genes. J. Immunol. 160:1320-1329. - PubMed
-
- Dzata, G. K., A. W. Confer, and J. H. Wyckoff. 1991. The effects of adjuvants on immune responses in cattle injected with a Brucella abortus soluble antigen. Vet. Microbiol. 29:27-48. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

