Observational study of upper gastrointestinal haemorrhage in elderly patients given selective cyclo-oxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs
- PMID: 12242172
- PMCID: PMC126302
- DOI: 10.1136/bmj.325.7365.624
Observational study of upper gastrointestinal haemorrhage in elderly patients given selective cyclo-oxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs
Abstract
Objective: To compare rates of upper gastrointestinal haemorrhage among elderly patients given selective cyclo-oxygenase-2 (COX 2) inhibitors and non-selective non-steroidal anti-inflammatory drugs (NSAIDs).
Design: Observational cohort study.
Setting: Administrative data from Ontario, Canada, used from 17 April 2000 to 31 March 2001 to identify population based, NSAID-naive cohorts of patients.
Patients: Subjects aged > or =66 years who started taking non-selective NSAIDs (n=5391), diclofenac plus misoprostol (n=5087), rofecoxib (n=14 583), or celecoxib (n=18 908) and a randomly selected control cohort not exposed to NSAIDs (n=100 000).
Main outcome measures: Rate ratios of hospital admission for upper gastrointestinal haemorrhage in each drug cohort with adjustment for potential confounders.
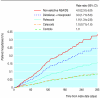
Results: Relative to controls, the multivariate model revealed an increased short term risk of upper gastrointestinal haemorrhage for users of non-selective NSAIDs (adjusted rate ratio 4.0 (95% confidence intervals 2.3 to 6.9)), diclofenac plus misoprostol (3.0 (1.7 to 5.6)), and rofecoxib (1.9 (1.3 to 2.8)) but not celecoxib (1.0 (0.7 to 1.6)). Relative to celecoxib, significantly higher risks of upper gastrointestinal haemorrhage were observed for non-selective NSAIDs (4.4 (2.3 to 8.5)), diclofenac plus misoprostol (3.2 (1.6 to 6.5)), and rofecoxib (1.9 (1.2 to 2.8)). Relative to rofecoxib, non-selective NSAID users were at significantly higher risk of upper gastrointestinal haemorrhage (1.9 (1.0 to 3.5)).
Conclusions: This population based observational study found a lower short term risk of upper gastrointestinal haemorrhage for selective COX-2 inhibitors compared with non-selective NSAIDs.
Figures
Comment in
-
Efficacy and safety of COX 2 inhibitors.BMJ. 2002 Sep 21;325(7365):607-8. doi: 10.1136/bmj.325.7365.607. BMJ. 2002. PMID: 12242157 Free PMC article. No abstract available.
References
-
- Misoprostol for co-prescription with NSAIDs. Drug Ther Bull. 1990;28:25–26. - PubMed
-
- Barat I, Andreasen F, Damsgaard EMS. The consumption of drugs by 75-year-old individuals living in their own homes. Eur J Clin Pharmacol. 2000;56:501–509. - PubMed
-
- Sayer GP, Britt H, Horn F, Bhasale A, McGeechan K, Charles J, et al. Measures of health and health care delivery in general practice in Australia. Australian Institute of Health and Welfare. April 2000. ( www.aihw.gov.au/publications/health/mhhcdgpa/index.html (accessed 27 May 2002))
-
- Hawkey CJ. COX-2 inhibitors. Lancet. 1999;353:307–314. - PubMed
-
- IMS Health Canada. New arthritis medication achieves fastest adoption ever recorded in Canada. Newsletter September 1999. ( www.imshealthcanada.com/htmen/4_2_1_14.htm)
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous