The adapter protein ZIP binds Grb14 and regulates its inhibitory action on insulin signaling by recruiting protein kinase Czeta
- PMID: 12242277
- PMCID: PMC139806
- DOI: 10.1128/MCB.22.20.6959-6970.2002
The adapter protein ZIP binds Grb14 and regulates its inhibitory action on insulin signaling by recruiting protein kinase Czeta
Abstract
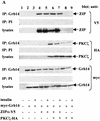
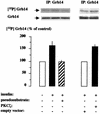
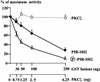
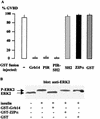
Grb14 is a member of the Grb7 family of adapters and acts as a negative regulator of insulin-mediated signaling. Here we found that the protein kinase Czeta (PKCzeta) interacting protein, ZIP, interacted with Grb14. Coimmunoprecipitation experiments demonstrated that ZIP bound to both Grb14 and PKCzeta, thereby acting as a link in the assembly of a PKCzeta-ZIP-Grb14 heterotrimeric complex. Mapping studies indicated that ZIP interacted through its ZZ zinc finger domain with the phosphorylated insulin receptor interacting region (PIR) of Grb14. PKCzeta phosphorylated Grb14 under in vitro conditions and in CHO-IR cells as demonstrated by in vivo labeling experiments. Furthermore, Grb14 phosphorylation was increased under insulin stimulation, suggesting that the PKCzeta-ZIP-Grb14 complex is involved in insulin signaling. The PIR of Grb14, which also interacts with the catalytic domain of the insulin receptor (IR) and inhibits its activity, was preferentially phosphorylated by PKCzeta. Interestingly, the phosphorylation of Grb14 by PKCzeta increased its inhibitory effect on IR tyrosine kinase activity in vitro. The role of ZIP and Grb14 in insulin signaling was further investigated in vivo in Xenopus laevis oocytes. In this model, ZIP potentiated the inhibitory action of Grb14 on insulin-induced oocyte maturation. Importantly, this effect required the recruitment of PKCzeta and the phosphorylation of Grb14, providing in vivo evidences for a regulation of Grb14-inhibitory action by ZIP and PKCzeta. Together, these results suggest that Grb14, ZIP, and PKCzeta participate in a new feedback pathway of insulin signaling.
Figures








References
-
- Bandyopadhyay, G., M. L. Standaert, U. Kikkawa, Y. Ono, J. Moscat, and R. V. Farese. 1999. Effect of transiently expressed atypical (zeta, lambda), conventional (alpha, beta) and novel (delta, epsilon) protein kinase C isoforms on insulin-stimulated translocation of epitope-tagged GLUT4 glucose transporters in rat adipocytes: specific interchangeable effects of protein kinase C-zeta and C-lambda. Biochem. J. 337:461-470. - PMC - PubMed
-
- Bereziat, V., A. Kasus-Jacobi, D. Perdereau, B. Cariou, J. Girard, and A. F. Burnol. 2002. Inhibition of insulin receptor catalytic activity by the molecular adapter Grb14. J. Biol. Chem. 28:4845-4852. - PubMed
-
- Berra, E., M. T. Diaz-Meco, I. Dominguez, M. M. Municio, L. Sanz, J. Lozano, R. S. Chapkin, and J. Moscat. 1993. Protein kinase C zeta isoform is critical for mitogenic signal transduction. Cell 74:555-563. - PubMed
-
- Bossenmaier, B., L. Mosthaf, H. Mischak, A. Ullrich, and H. U. Haring. 1997. Protein kinase C isoforms beta 1 and beta 2 inhibit the tyrosine kinase activity of the insulin receptor. Diabetologia 40:863-866. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
