Pax3-FKHR knock-in mice show developmental aberrations but do not develop tumors
- PMID: 12242297
- PMCID: PMC139793
- DOI: 10.1128/MCB.22.20.7204-7216.2002
Pax3-FKHR knock-in mice show developmental aberrations but do not develop tumors
Abstract
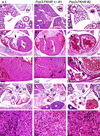
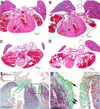
Alveolar rhabdomyosarcoma is a pediatric disease specified by the recurrent chromosome translocations t(2;13) and t(1;13). These translocations result in the formation of the PAX3-FKHR and PAX7-FKHR fusion genes, which are thought to play a causal role in the genesis of this disease. Although PAX3-FKHR exhibits transforming activity in immortalized fibroblast cell lines, a direct role of this fusion protein in tumorigenesis in vivo has not been shown. We determined whether expression of Pax3-FKHR in the mouse germ line would render these animals prone to the development of rhabdomyosarcomas. By targeting FKHR cDNA sequences into the Pax3 locus of embryonic stem cells, we used these cells to generate mice carrying a Pax3-FKHR knock-in allele. Despite low expression of the knock-in allele, heterozygous offspring of Pax3-FKHR chimeric mice showed developmental abnormalities. These included intraventricular septum defects, tricuspid valve insufficiency, and diaphragm defects, which caused congestive heart failure leading to perinatal death. In addition, Pax3-FKHR heterozygous offspring displayed malformations of some but not all hypaxial muscles. However, neither newborn heterozygous pups nor their chimeric parents showed any signs of malignancy. We conclude that the Pax3-FKHR allele causes lethal developmental defects in knock-in mice but might be insufficient to cause muscle tumors.
Figures



References
-
- Barr, F. G., N. Galili, J. Holick, J. A. Biegel, G. Rovera, and B. S. Emanuel. 1993. Rearrangement of the PAX3 paired box gene in the paediatric solid tumour alveolar rhabdomyosarcoma. Nat. Genet. 3:113-117. - PubMed
-
- Bennicelli, J. L., S. Advani, B. W. Schafer, and F. G. Barr. 1999. PAX3 and PAX7 exhibit conserved cis-acting transcription repression domains and utilize a common gain of function mechanism in alveolar rhabdomyosarcoma. Oncogene 18:4348-4356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
