Endosomal signaling of epidermal growth factor receptor stimulates signal transduction pathways leading to cell survival
- PMID: 12242303
- PMCID: PMC139821
- DOI: 10.1128/MCB.22.20.7279-7290.2002
Endosomal signaling of epidermal growth factor receptor stimulates signal transduction pathways leading to cell survival
Abstract
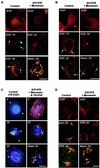
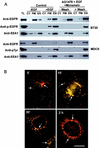
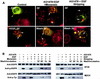
In spite of intensified efforts to understand cell signaling from endosomes, there is no direct evidence demonstrating that endosomal signaling is sufficient to activate signal transduction pathways and no evidence to demonstrate that endosomal signaling is able to produce a biological outcome. The lack of breakthrough is due in part to the lack of means to generate endosomal signals without plasma membrane signaling. In this paper, we report the establishment of a system to specifically activate epidermal growth factor (EGF) receptor (EGFR) when it endocytoses into endosomes. We treated cells with EGF in the presence of AG-1478, a specific EGFR tyrosine kinase inhibitor, and monensin, which blocks the recycling of EGFR. This treatment led to the internalization of nonactivated EGF-EGFR complexes into endosomes. The endosome-associated EGFR was then activated by removing AG-1478 and monensin. During this procedure we did not observe any surface EGFR phosphorylation. We also achieved specific activation of endosome-associated EGFR without using monensin. By using this system, we provided original evidence demonstrating that (i) the endosome can serve as a nucleation site for the formation of signaling complexes, (ii) endosomal EGFR signaling is sufficient to activate the major signaling pathways leading to cell proliferation and survival, and (iii) endosomal EGFR signaling is sufficient to suppress apoptosis induced by serum withdrawal.
Figures

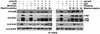

Similar articles
-
Internalization of inactive EGF receptor into endosomes and the subsequent activation of endosome-associated EGF receptors. Epidermal growth factor.Sci STKE. 2002 Dec 3;2002(161):pl17. doi: 10.1126/stke.2002.161.pl17. Sci STKE. 2002. PMID: 12464704
-
Activation of Endosome-Associated Inert EGF Receptor Following Internalization.Methods Mol Biol. 2017;1652:117-126. doi: 10.1007/978-1-4939-7219-7_8. Methods Mol Biol. 2017. PMID: 28791638
-
Stimulation of cell proliferation by endosomal epidermal growth factor receptor as revealed through two distinct phases of signaling.Mol Cell Biol. 2003 Aug;23(16):5803-15. doi: 10.1128/MCB.23.16.5803-5815.2003. Mol Cell Biol. 2003. PMID: 12897150 Free PMC article.
-
The physical basis of analog-to-digital signal processing in the EGFR system-Delving into the role of the endoplasmic reticulum.Bioessays. 2024 Sep;46(9):e2400026. doi: 10.1002/bies.202400026. Epub 2024 Jul 11. Bioessays. 2024. PMID: 38991978 Review.
-
Trafficking of the ErbB receptors and its influence on signaling.Exp Cell Res. 2003 Mar 10;284(1):78-88. doi: 10.1016/s0014-4827(03)00002-8. Exp Cell Res. 2003. PMID: 12648467 Review.
Cited by
-
Phosphatidic Acid Increases Epidermal Growth Factor Receptor Expression by Stabilizing mRNA Decay and by Inhibiting Lysosomal and Proteasomal Degradation of the Internalized Receptor.Mol Cell Biol. 2015 Sep;35(18):3131-44. doi: 10.1128/MCB.00286-15. Epub 2015 Jun 29. Mol Cell Biol. 2015. PMID: 26124282 Free PMC article.
-
The endosomal adaptor protein APPL1 impairs the turnover of leading edge adhesions to regulate cell migration.Mol Biol Cell. 2012 Apr;23(8):1486-99. doi: 10.1091/mbc.E11-02-0124. Epub 2012 Feb 29. Mol Biol Cell. 2012. PMID: 22379109 Free PMC article.
-
HCMV-induced signaling through gB-EGFR engagement is required for viral trafficking and nuclear translocation in primary human monocytes.Proc Natl Acad Sci U S A. 2020 Aug 11;117(32):19507-19516. doi: 10.1073/pnas.2003549117. Epub 2020 Jul 28. Proc Natl Acad Sci U S A. 2020. PMID: 32723814 Free PMC article.
-
RhoB regulates PDGFR-beta trafficking and signaling in vascular smooth muscle cells.Arterioscler Thromb Vasc Biol. 2007 Dec;27(12):2597-605. doi: 10.1161/ATVBAHA.107.154211. Epub 2007 Oct 19. Arterioscler Thromb Vasc Biol. 2007. PMID: 17951322 Free PMC article.
-
PGE2 mediates EGFR internalization and nuclear translocation via caveolin endocytosis promoting its transcriptional activity and proliferation in human NSCLC cells.Oncotarget. 2018 Feb 15;9(19):14939-14958. doi: 10.18632/oncotarget.24499. eCollection 2018 Mar 13. Oncotarget. 2018. PMID: 29599917 Free PMC article.
References
-
- Ahn, S., S. Maudsley, L. M. Luttrell, R. J. Lefkowitz, and Y. Daaka. 1999. Src-mediated tyrosine phosphorylation of dynamin is required for beta2-adrenergic receptor internalization and mitogen-activated protein kinase signaling. J. Biol. Chem. 274:1185-1188. - PubMed
-
- Burgering, B. M., and P. J. Coffer. 1995. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature 376:599-602. - PubMed
-
- Carpenter, G. 1987. Receptors for epidermal growth factor and other polypeptide mitogens. Annu. Rev. Biochem. 56:881-914. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
