Mouse Af9 is a controller of embryo patterning, like Mll, whose human homologue fuses with Af9 after chromosomal translocation in leukemia
- PMID: 12242306
- PMCID: PMC139815
- DOI: 10.1128/MCB.22.20.7313-7324.2002
Mouse Af9 is a controller of embryo patterning, like Mll, whose human homologue fuses with Af9 after chromosomal translocation in leukemia
Abstract
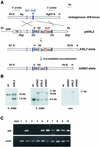
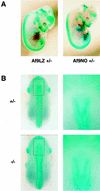
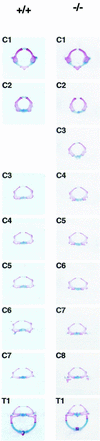
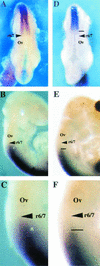
Chromosomal translocation t(9;11)(p22;q23) in acute myeloid leukemia fuses the MLL and AF9 genes. We have inactivated the murine homologue of AF9 to elucidate its normal role. No effect on hematopoiesis was observed in mice with a null mutation of Af9. However, an Af9 null mutation caused perinatal lethality, and homozygous mice exhibited anomalies of the axial skeleton. Both the cervical and thoracic regions were affected by anterior homeotic transformation. Strikingly, mice lacking functional Af9 exhibited a grossly deformed atlas and an extra cervical vertebra. To determine the molecular mediators of this phenotype, analysis of Hox gene expression by in situ hybridization showed that Af9 null embryos have posterior changes in Hoxd4 gene expression. We conclude that the Af9 gene is required for normal embryogenesis in mice by controlling pattern formation, apparently via control of Hox gene regulation. This is analogous to the role of Mll, the murine homolog of human MLL, to which the Af9 gene fuses in acute myeloid leukemias.
Figures



References
-
- Ayton, P. M., and M. L. Cleary. 2001. Molecular mechanisms of leukemogenesis mediated by MLL fusion proteins. Oncogene Rev. 20:5695-5707. - PubMed
-
- Breen, T. R., and P. J. Harte. 1993. Trithorax regulates multiple homeotic genes in the bithorax and Antennapedia complexes and exerts different tissue-specific, parasegment-specific and promoter-specific effects on each. Development 117:119-134. - PubMed
-
- Broeker, P. L., H. G. Super, M. J. Thirman, H. Pomykala, Y. Yonebayashi, S. Tanabe, N. Zeleznik-Le, and J. D. Rowley. 1996. Distribution of 11q23 breakpoints within the MLL breakpoint cluster region in de novo acute leukemia and in treatment-related acute myeloid leukemia: correlation with scaffold attachment regions and topoisomerase II consensus binding sites. Blood 87:1912-1922. - PubMed
-
- Buske, C., and R. K. Humphries. 2000. Homeobox genes in leukemogenesis. Int. J. Hematol. 71:301-308. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
