COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression
- PMID: 12242329
- PMCID: PMC129799
- DOI: 10.1073/pnas.162468699
COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression
Abstract
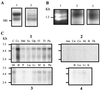
Two cyclooxygenase isozymes, COX-1 and -2, are known to catalyze the rate-limiting step of prostaglandin synthesis and are the targets of nonsteroidal antiinflammatory drugs. Here we describe a third distinct COX isozyme, COX-3, as well as two smaller COX-1-derived proteins (partial COX-1 or PCOX-1 proteins). COX-3 and one of the PCOX-1 proteins (PCOX-1a) are made from the COX-1 gene but retain intron 1 in their mRNAs. PCOX-1 proteins additionally contain an in-frame deletion of exons 5-8 of the COX-1 mRNA. COX-3 and PCOX mRNAs are expressed in canine cerebral cortex and in lesser amounts in other tissues analyzed. In human, COX-3 mRNA is expressed as an approximately 5.2-kb transcript and is most abundant in cerebral cortex and heart. Intron 1 is conserved in length and in sequence in mammalian COX-1 genes. This intron contains an ORF that introduces an insertion of 30-34 aa, depending on the mammalian species, into the hydrophobic signal peptide that directs COX-1 into the lumen of the endoplasmic reticulum and nuclear envelope. COX-3 and PCOX-1a are expressed efficiently in insect cells as membrane-bound proteins. The signal peptide is not cleaved from either protein and both proteins are glycosylated. COX-3, but not PCOX-1a, possesses glycosylation-dependent cyclooxygenase activity. Comparison of canine COX-3 activity with murine COX-1 and -2 demonstrates that this enzyme is selectively inhibited by analgesic/antipyretic drugs such as acetaminophen, phenacetin, antipyrine, and dipyrone, and is potently inhibited by some nonsteroidal antiinflammatory drugs. Thus, inhibition of COX-3 could represent a primary central mechanism by which these drugs decrease pain and possibly fever.
Figures
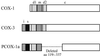



Comment in
-
Cyclooxygenase-3 (COX-3): filling in the gaps toward a COX continuum?Proc Natl Acad Sci U S A. 2002 Oct 15;99(21):13371-3. doi: 10.1073/pnas.222543099. Epub 2002 Oct 8. Proc Natl Acad Sci U S A. 2002. PMID: 12374850 Free PMC article. Review. No abstract available.
-
COX-3: in the wrong frame in mind.Immunol Lett. 2003 Mar 3;86(1):121. doi: 10.1016/s0165-2478(02)00268-7. Immunol Lett. 2003. PMID: 12600754 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

