Aberrant accumulation of EFEMP1 underlies drusen formation in Malattia Leventinese and age-related macular degeneration
- PMID: 12242346
- PMCID: PMC130587
- DOI: 10.1073/pnas.202491599
Aberrant accumulation of EFEMP1 underlies drusen formation in Malattia Leventinese and age-related macular degeneration
Abstract
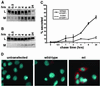
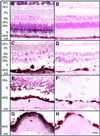
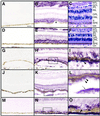
Malattia Leventinese (ML), an inherited macular degenerative disease, is closely reminiscent of age-related macular degeneration (AMD), the most common cause of incurable blindness. Both ML and AMD are characterized by extracellular deposits known as drusen between the retinal pigment epithelium (RPE) and Bruch's membrane. The mechanism underlying drusen formation is unknown. An Arg to Trp mutation in a gene of unknown function, EFEMP1, is responsible for ML, indicating EFEMP1 may be important in drusen formation. Here, we show that wild-type EFEMP1 is a secreted protein whereas mutant EFEMP1 is misfolded, secreted inefficiently, and retained within cells. In normal eyes, EFEMP1 is not present at the site of drusen formation. However, in ML eyes, EFEMP1 accumulates within the RPE cells and between the RPE and drusen, but does not appear to be a major component of drusen. Furthermore, in AMD eyes, EFEMP1 is found to accumulate beneath the RPE immediately overlaying drusen, but not in the region where there is no apparent retinal pathology observed. These data present evidence that misfolding and aberrant accumulation of EFEMP1 may cause drusen formation and cellular degeneration and play an important role in the etiology of both ML and AMD.
Figures

References
-
- Doyne R W. Trans Ophthalmol Soc UK. 1899;19:71.
-
- Vogt A. In: Untersuchungsmethoden. 3rd Ed. Graefe A, Saemisch T, editors. Berlin: Verlag von Wilhelm Engelman; 1925. pp. 1–118.
-
- Klainguti R. Klin Monatsbl Augenheikd. 1932;89:253–254.
-
- Evans K, Gregory C Y, Wijesuriya S D, Kermani S, Jay M R, Plant C, Bird A C. Arch Ophthalmol. 1997;115:904–910. - PubMed
-
- Collins T. Ophthalmoscope. 1913;11:537–538.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

