Cell type-specific responses of human cells to inhibition of replication licensing
- PMID: 12242660
- PMCID: PMC3605503
- DOI: 10.1038/sj.onc.1205910
Cell type-specific responses of human cells to inhibition of replication licensing
Abstract
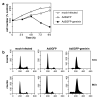
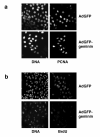
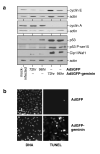
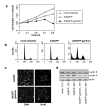
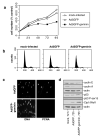
Replication origins are 'licensed' for a single initiation event by loading Mcm2-7 complexes during late mitosis and G1. Licensing is blocked at other cell cycle stages by the activity of cyclin-dependent kinases and a small protein called geminin. Here, we describe the effects of over-expressing a non-degradable form of geminin in various cell lines. Geminin expression reduced the quantity of Mcm2 bound to chromatin and blocked cell proliferation. U2OS (p53+/Rb+) cells showed an early S phase arrest with high cyclin E and undetectable cyclin A levels, consistent with the activation of an intra-S checkpoint. Saos2 (p53-/Rb-) cells showed an accumulation of cells in late S and G2/M with approximately normal levels of cyclin A, consistent with loss of this intra-S phase checkpoint. Geminin also induced apoptosis in both these cell lines. In contrast, IMR90 primary fibroblasts over-expressing geminin arrested in G1 with reduced cyclin E levels and no detectable apoptosis. A 'licensing checkpoint' may therefore act in primary cells to prevent passage into S phase in the absence of sufficient origin licensing. These results suggest that inhibition of the licensing system may cause cancer-specific cell killing and therefore represent a novel anti-cancer target.
Figures
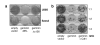


References
-
- Abraham RT. Genes Dev. 2001;15:2177–2196. - PubMed
-
- Arentson E, Faloon P, Seo J, Moon E, Studts JM, Fremont DH, Choi K. Oncogene. 2002;21:1150–1158. - PubMed
-
- Blow JJ, Laskey RA. Nature. 1988;332:546–548. - PubMed
-
- Burkhart R, Schulte D, Hu D, Musahl C, Gohring F, Knippers R. Eur. J. Biochem. 1995;228:431–438. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

