Distinct antigenic features of linear epitopes at the N-terminus and C-terminus of 65 kDa glutamic acid decarboxylase (GAD65): implications for autoantigen modification during pathogenesis
- PMID: 12296864
- PMCID: PMC1906485
- DOI: 10.1046/j.1365-2249.2002.01960.x
Distinct antigenic features of linear epitopes at the N-terminus and C-terminus of 65 kDa glutamic acid decarboxylase (GAD65): implications for autoantigen modification during pathogenesis
Abstract
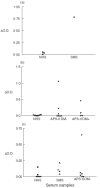
Autoantibodies to 65 kDa glutamic acid decarboxylase (GAD65) are produced in many patients with autoimmune polyendocrine syndrome type II (APS-II) or stiff-man syndrome (SMS) and are heterogeneous in their epitope specificities, recognizing both conformational and linear determinants. Major linear epitopes of GAD, which are recognized by autoantibodies in a minority of these patients, occur in the N-terminal and C-terminal regions. We have investigated antibody recognition of the N- and C-termini of GAD65 in relation to their structural features as an approach to understanding what modifications to the native GAD structure may occur that facilitate the generation of antibodies specific to linear epitopes in these regions during the autoimmune pathogenesis. A monoclonal antibody specific to the N-terminus of GAD65 bound both native and denatured GAD in ELISA, whereas monoclonal and polyclonal antibodies specific to the C-terminus of GAD bound only denatured GAD. These antibodies were epitope mapped using random peptide phage-display libraries and the epitopes related to a previously proposed structural model of GAD65. This has led us to propose that the alpha-helical secondary structure of the C-terminus of GAD65 must be denatured to generate linear epitopes. In contrast, the N-terminus is both surface exposed and linear in the native structure, but may be masked by membrane interactions, which must be broken to facilitate recognition by B cells.
Figures



References
-
- Leslie RDG, Atkinson MA, Notkins AL. Autoantigens IA-2 and GAD in type 1 (insulin-dependent) diabetes. Diabetologia. 1999;42:3–14. - PubMed
-
- Kanaani J, Lissin D, Kash SF, Baekkeskov S. The hydrophilic isoform of glutamate decarboxylase, GAD67, is targeted to membranes and nerve terminals independent of dimerization with the hydrophilic membrane-anchored isoform, GAD65. J Biol Chem. 1999;274:37200–9. - PubMed
-
- Bonifacio E, Lampasona V, Bernasconi L, Ziegler A-G. Maturation of the humoral autoimmune response to epitopes of GAD in preclinical childhood type 1 diabetes. Diabetes. 2000;49:202–8. - PubMed
-
- Solimena M, Folli F, Denis-Donini S. Autoantibodies to glutamate decarboxylase in a patient with stiff man syndrome, epilepsy and type 1 diabetes mellitus. N Engl J Med. 1988;318:1012–20. - PubMed
-
- Baekkeskov S, Aanstoot HJ, Christgau S, et al. Identification of the 64k autoantigen in insulin-dependent diabetes as the GABA synthesizing enzyme glutamic acid decarboxylase. Nature. 1990;347:151–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

