Mediators of inflammation are down-regulated while apoptosis is up-regulated in rheumatoid arthritis synovial tissue by polymerized collagen
- PMID: 12296865
- PMCID: PMC1906486
- DOI: 10.1046/j.1365-2249.2002.01955.x
Mediators of inflammation are down-regulated while apoptosis is up-regulated in rheumatoid arthritis synovial tissue by polymerized collagen
Erratum in
- Clin Exp Immunol 2002 Dec;130(3):565-6
Abstract
The aim of the study was to determine whether collagen-polyvinylpyrrolidone (collagen-PVP) modifies some proinflammatory responses in synovium cultures from rheumatoid arthritis (RA) patients. Synovium from 10 RA patients were cultured with or without 1% collagen-PVP. Tissues on the 3rd, 5th and 7th culture day were sectioned and stained by the Herovici technique. Total collagen and type I/III collagen ratios were evaluated by the Woessner micromethod and by interrupted gel electrophoresis, respectively. Collagenolytic activity was assessed by degradation of [3H]-collagen in supernatants. TIMP-1, IL-1beta and TNF-alpha were determined in supernatants by ELISA, and the results were normalized by DNA concentration. IL-1beta, TNF-alpha, IL-6, IL-8, MMP-1, TIMP-1, Cox-1, VCAM-1, ICAM-1 and Fas/APO95 expression was evaluated by immunohistochemistry. Apoptosis was detected by TUNEL technique. The histological analysis and electrophoresis revealed a 1.7-fold increase of type III collagen in a time-dependent fashion in collagen-PVP-treated cultures. Proinflammatory cytokines (IL-1beta: 58 +/- 9 versus 22 +/- 10; TNF-alpha: 41 +/- 6 versus 11 +/- 3; IL-8: 59 +/- 12 versus 29 +/- 9; treated versus untreated), adhesion molecule (ICAM-1: 57 +/- 11 versus 29 +/- 15; VCAM-1: 49 +/- 7 versus 21 +/- 13; treated versus untreated) as well as Cox-1 (59 +/- 10 versus 20 +/- 3) expression was down-regulated in RA synovium treated. Meanwhile, TIMP-1 (36 +/- 7 versus 57 +/- 11) and Fas expression (20 +/- 10 versus 55 +/- 13) and apoptosis (14 +/- 3 versus 55 +/- 5) were up-regulated in treated cultures compared with controls. In supernatants, the collagenolytic activity, as well as IL-1beta and TNF-alpha, levels were all down-regulated in treated cultures (two, three, fourfold, respectively). The addition of collagen-PVP to synovium-induced down-modulation of some inflammatory parameters and an increase in apoptosis of synovial cells. Perhaps this mechanism could contribute to inhibit outgrowth of pannus formation and to down-regulate inflammation of joints in patients with RA.
Figures
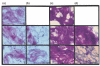
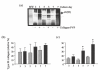
 , Type III collagen in control group; ▪, Type III collagen in collagen-PVP group.
, Type III collagen in control group; ▪, Type III collagen in collagen-PVP group.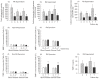
 , control; ▪, collagen-PVP.
, control; ▪, collagen-PVP.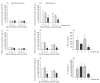
 , control; ▪, collagen-PVP.
, control; ▪, collagen-PVP.References
-
- Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–439. - PubMed
-
- Nakajima T, Aono H, Hasunuma T, et al. Apoptosis and functional Fas antigen in rheumatoid arthritis synoviocytes. Arthritis Rheum. 1995;38:485–91. - PubMed
-
- Tsuboi M, Eguchi K, Kawakami K, et al. Fas antigen expression on synovial cells was down-regulated by interleukin 1β. Biochem Biophys Res Commun. 1996;218:280–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

