Anaerobic oxidation of arsenite in Mono Lake water and by a facultative, arsenite-oxidizing chemoautotroph, strain MLHE-1
- PMID: 12324322
- PMCID: PMC126446
- DOI: 10.1128/AEM.68.10.4795-4802.2002
Anaerobic oxidation of arsenite in Mono Lake water and by a facultative, arsenite-oxidizing chemoautotroph, strain MLHE-1
Abstract
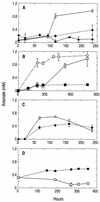
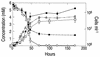
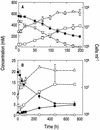
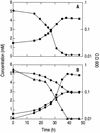
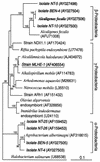
Arsenite [As(III)]-enriched anoxic bottom water from Mono Lake, California, produced arsenate [As(V)] during incubation with either nitrate or nitrite. No such oxidation occurred in killed controls or in live samples incubated without added nitrate or nitrite. A small amount of biological As(III) oxidation was observed in samples amended with Fe(III) chelated with nitrolotriacetic acid, although some chemical oxidation was also evident in killed controls. A pure culture, strain MLHE-1, that was capable of growth with As(III) as its electron donor and nitrate as its electron acceptor was isolated in a defined mineral salts medium. Cells were also able to grow in nitrate-mineral salts medium by using H(2) or sulfide as their electron donor in lieu of As(III). Arsenite-grown cells demonstrated dark (14)CO(2) fixation, and PCR was used to indicate the presence of a gene encoding ribulose-1,5-biphosphate carboxylase/oxygenase. Strain MLHE-1 is a facultative chemoautotroph, able to grow with these inorganic electron donors and nitrate as its electron acceptor, but heterotrophic growth on acetate was also observed under both aerobic and anaerobic (nitrate) conditions. Phylogenetic analysis of its 16S ribosomal DNA sequence placed strain MLHE-1 within the haloalkaliphilic Ectothiorhodospira of the gamma-PROTEOBACTERIA: Arsenite oxidation has never been reported for any members of this subgroup of the PROTEOBACTERIA:
Figures


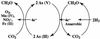
References
-
- Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. - PubMed
-
- Banin, A., B. C. Clark, and H. Wanke. 1992. Surface chemistry and mineralogy, p. 594-625. In H. H. Kieffer, B. M. Jakowsky, C. W. Snyder, and M. S. Matthews (ed.), Mars. University of Arizona Press, Tucson.
-
- Bard, A. J., R. Parsons, and J. Jordon. 1985. Standard potentials in aqueous solutions. Marcel Dekker, New York, N.Y.
-
- Cervantes, C., G. Ji, J. L. Ramírez, and S. Silver. 1994. Resistance to arsenic compounds in microorganisms. FEMS Microbiol. Rev. 15:355-367. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

