Diversity of endophytic bacterial populations and their interaction with Xylella fastidiosa in citrus plants
- PMID: 12324338
- PMCID: PMC126398
- DOI: 10.1128/AEM.68.10.4906-4914.2002
Diversity of endophytic bacterial populations and their interaction with Xylella fastidiosa in citrus plants
Abstract
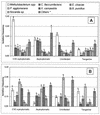
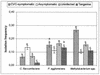
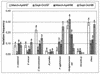
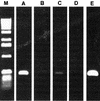
Citrus variegated chlorosis (CVC) is caused by Xylella fastidiosa, a phytopathogenic bacterium that can infect all Citrus sinensis cultivars. The endophytic bacterial communities of healthy, resistant, and CVC-affected citrus plants were studied by using cultivation as well as cultivation-independent techniques. The endophytic communities were assessed in surface-disinfected citrus branches by plating and denaturing gradient gel electrophoresis (DGGE). Dominant isolates were characterized by fatty-acid methyl ester analysis as Bacillus pumilus, Curtobacterium flaccumfaciens, Enterobacter cloacae, Methylobacterium spp. (including Methylobacterium extorquens, M. fujisawaense, M. mesophilicum, M. radiotolerans, and M. zatmanii), Nocardia sp., Pantoea agglomerans, and Xanthomonas campestris. We observed a relationship between CVC symptoms and the frequency of isolation of species of Methylobacterium, the genus that we most frequently isolated from symptomatic plants. In contrast, we isolated C. flaccumfaciens significantly more frequently from asymptomatic plants than from those with symptoms of CVC while P. agglomerans was frequently isolated from tangerine (Citrus reticulata) and sweet-orange (C. sinensis) plants, irrespective of whether the plants were symptomatic or asymptomatic or showed symptoms of CVC. DGGE analysis of 16S rRNA gene fragments amplified from total plant DNA resulted in several bands that matched those from the bacterial isolates, indicating that DGGE profiles can be used to detect some endophytic bacteria of citrus plants. However, some bands had no match with any isolate, suggesting the occurrence of other, nonculturable or as yet uncultured, endophytic bacteria. A specific band with a high G+C ratio was observed only in asymptomatic plants. The higher frequency of C. flaccumfaciens in asymptomatic plants suggests a role for this organism in the resistance of plants to CVC.
Figures




References
-
- Araújo, W. L., H. O. Saridakis, P. A. V. Barroso, C. I. Aguilar-Vildoso, and J. L. Azevedo. 2001. Variability and interactions between endophytic bacteria and fungi isolated from leaf tissues of citrus rootstocks. Can. J. Microbiol. 47:229-236. - PubMed
-
- Azevedo, J. L., W. Maccheroni, Jr., J. O. Pereira, and W. L. Araújo. 15April2000, posting date. Endophytic microorganisms: a review on insect control and recent advances on tropical plants. Bio/Technology 3:40-65. [Online.] http://www.ejb.org/content/vol3/issue1/full/3/4.
-
- Bent, E., and C. P. Chanway. 1998. The growth-promoting effects of a bacterial endophyte on lodgepole pine are partially inhibited by the presence of other rhizobacteria. Can. J. Microbiol. 44:980-988.
-
- Chang, C. J., M. Garnier, L. Zreik, V. Rossetti, and J. M. Bove. 1993. Culture and serological detection of the xylem-limited bacterium causing citrus variegated chlorosis and its identification as a strain of Xylella fastidiosa. Curr. Microbiol. 27:137-142. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

